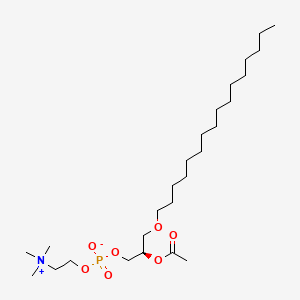
Platelet activating factor
Platelet activating factor is a lipid of Glycerophospholipids (GP) class. Platelet activating factor is associated with abnormalities such as Atherosclerosis, Acute cholecystitis without calculus, Cholecystitis, Colitis and Cholecystitis, Acute. The involved functions are known as Cell Survival, Metabolic Inhibition, lipid oxidation, Apoptosis and Oxidation. Platelet activating factor often locates in soluble, Cellular Membrane, Smooth muscle (tissue), Intima and Tissue specimen. The associated genes with Platelet activating factor are apolipoprotein A-I Milano, Homologous Gene, TSPO gene, HBEGF gene and SLC33A1 gene. The related lipids are Hydroxycholesterols, Liposomes, 25-hydroxycholesterol, Lysophosphatidylcholines and Lipopolysaccharides. The related experimental models are Knock-out, Mouse Model and Transgenic Model.
Cross Reference
Introduction
To understand associated biological information of Platelet activating factor, we collected biological information of abnormalities, associated pathways, cellular/molecular locations, biological functions, related genes/proteins, lipids and common seen animal/experimental models with organized paragraphs from literatures.
What diseases are associated with Platelet activating factor?
Platelet activating factor is suspected in Ischemia, Pleurisy, Atherosclerosis, Inflammatory disorder, Retinal Diseases, Diabetes and other diseases in descending order of the highest number of associated sentences.
Related references are mostly published in these journals:
- J. Lipid Res. (5)
- Am. J. Physiol., Cell Physiol. (2)
- Arterioscler. Thromb. Vasc. Biol. (2)
- Others (26)
| Disease | Cross reference | Weighted score | Related literature |
|---|
Possible diseases from mapped MeSH terms on references
We collected disease MeSH terms mapped to the references associated with Platelet activating factor
PubChem Associated disorders and diseases
What pathways are associated with Platelet activating factor
There are no associated biomedical information in the current reference collection.
PubChem Biomolecular Interactions and Pathways
Link to PubChem Biomolecular Interactions and PathwaysWhat cellular locations are associated with Platelet activating factor?
Visualization in cellular structure
Associated locations are in red color. Not associated locations are in black.
Related references are published most in these journals:
| Location | Cross reference | Weighted score | Related literatures |
|---|
What functions are associated with Platelet activating factor?
Related references are published most in these journals:
| Function | Cross reference | Weighted score | Related literatures |
|---|
What lipids are associated with Platelet activating factor?
Related references are published most in these journals:
| Lipid concept | Cross reference | Weighted score | Related literatures |
|---|
What genes are associated with Platelet activating factor?
Related references are published most in these journals:
| Gene | Cross reference | Weighted score | Related literatures |
|---|
What common seen animal models are associated with Platelet activating factor?
Knock-out
Knock-out are used in the study 'A cardioprotective role for platelet-activating factor through NOS-dependent S-nitrosylation.' (Leary PJ et al., 2008).
Mouse Model
Mouse Model are used in the study 'A regulatory role of LPCAT1 in the synthesis of inflammatory lipids, PAF and LPC, in the retina of diabetic mice.' (Cheng L et al., 2009).
Transgenic Model
Transgenic Model are used in the study 'Heterogeneity in the sn-1 carbon chain of platelet-activating factor glycerophospholipids determines pro- or anti-apoptotic signaling in primary neurons.' (Ryan SD et al., 2008).
Related references are published most in these journals:
| Model | Cross reference | Weighted score | Related literatures |
|---|
NCBI Entrez Crosslinks
All references with Platelet activating factor
Download all related citations| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Fazal N et al. | PAF receptor antagonist modulates neutrophil responses with thermal injury in vivo. | 2001 | Am. J. Physiol., Cell Physiol. | pmid:11546669 |
| Roudebush WE and Mathur RS | Presence of platelet-activating factor in squirrel monkey (Saimiri boliviensis) spermatozoa: seasonal differences. | 1998 | Am. J. Primatol. | pmid:9651652 |
| Roudebush WE et al. | Relationship between platelet-activating factor concentration in rhesus monkey (Macaca mulatta) spermatozoa and sperm motility. | 2002 | Am. J. Primatol. | pmid:11793409 |
| Kawano Y et al. | The effects of platelet-activating factor on the secretion of interleukin-8 and growth-regulated oncogene alpha in human immortalized granulosa cell line (GC1a). | 2007 | Am. J. Reprod. Immunol. | pmid:17922696 |
| Elias KA et al. | Alteration in platelet count during early pregnancy in the mouse. | 1989 Nov-Dec | Am. J. Reprod. Immunol. | pmid:2640443 |
| Ripps BA et al. | Platelet-activating factor production from in vitro and in vivo fertilized murine embryos is similar. | 1993 Sep-Oct | Am. J. Reprod. Immunol. | pmid:8311917 |
| Minhas BS et al. | Platelet activating factor and conception. | 1996 | Am. J. Reprod. Immunol. | pmid:8962659 |
| Roudebush WE et al. | Effect of platelet-activating factor (PAF) on preimplantation mouse B6D2F1/J embryo formation. | 1996 | Am. J. Reprod. Immunol. | pmid:8962660 |
| Lash GE and Legge M | Localization and distribution of platelet activating factor receptors in the mouse ovary and oviduct during the estrous cycle and early pregnancy. | 2001 | Am. J. Reprod. Immunol. | pmid:11216875 |
| Roussev RG et al. | A novel bioassay for detection of preimplantation factor (PIF). | 1995 | Am. J. Reprod. Immunol. | pmid:7619236 |
| Ishizuka S et al. | Acid exposure stimulates the adherence of Streptococcus pneumoniae to cultured human airway epithelial cells: effects on platelet-activating factor receptor expression. | 2001 | Am. J. Respir. Cell Mol. Biol. | pmid:11306440 |
| Pawliczak R et al. | Oxidative stress induces arachidonate release from human lung cells through the epithelial growth factor receptor pathway. | 2002 | Am. J. Respir. Cell Mol. Biol. | pmid:12444032 |
| Samet JM et al. | Effect of ozone on platelet-activating factor production in phorbol-differentiated HL60 cells, a human bronchial epithelial cell line (BEAS S6), and primary human bronchial epithelial cells. | 1992 | Am. J. Respir. Cell Mol. Biol. | pmid:1419027 |
| Longphre M et al. | PAF-induced airways hyperreactivity is modulated by mast cells in mice. | 1996 | Am. J. Respir. Cell Mol. Biol. | pmid:8624251 |
| Okada S et al. | Migration of eosinophils through basement membrane components in vitro: role of matrix metalloproteinase-9. | 1997 | Am. J. Respir. Cell Mol. Biol. | pmid:9376127 |
| Block LH et al. | Platelet-activating factor (PAF)-dependent biochemical, morphologic, and physiologic responses of human platelets: demonstration of translocation of protein kinase C associated with protein phosphorylation. | 1989 | Am. J. Respir. Cell Mol. Biol. | pmid:2624764 |
| Guilbert M et al. | 5-Oxo-6,8,11,14-eicosatetraenoic acid induces important eosinophil transmigration through basement membrane components: comparison of normal and asthmatic eosinophils. | 1999 | Am. J. Respir. Cell Mol. Biol. | pmid:10385597 |
| McNulty CA et al. | Characterization of the integrin and activation steps mediating human eosinophil and neutrophil adhesion to chronically inflamed airway endothelium. | 1999 | Am. J. Respir. Cell Mol. Biol. | pmid:10340944 |
| Walker BA et al. | Adenosine A3 receptor expression and function in eosinophils. | 1997 | Am. J. Respir. Cell Mol. Biol. | pmid:9160835 |
| Tamm M et al. | Hypoxia-induced interleukin-6 and interleukin-8 production is mediated by platelet-activating factor and platelet-derived growth factor in primary human lung cells. | 1998 | Am. J. Respir. Cell Mol. Biol. | pmid:9761763 |
| Shirasaki H et al. | Expression of platelet-activating factor receptor mRNA in human and guinea pig lung. | 1994 | Am. J. Respir. Cell Mol. Biol. | pmid:8179916 |
| Henson PM | PAF--a perspective. | 1989 | Am. J. Respir. Cell Mol. Biol. | pmid:2696514 |
| Casale TB et al. | Platelet-activating factor-induced human eosinophil transendothelial migration: evidence for a dynamic role of the endothelium. | 1993 | Am. J. Respir. Cell Mol. Biol. | pmid:8380250 |
| Liu L et al. | Transmigration of human neutrophils across airway epithelial cell monolayers is preferentially in the physiologic basolateral-to-apical direction. | 1996 | Am. J. Respir. Cell Mol. Biol. | pmid:8969272 |
| Larivée P et al. | Platelet-activating factor induces airway mucin release via activation of protein kinase C: evidence for translocation of protein kinase C to membranes. | 1994 | Am. J. Respir. Cell Mol. Biol. | pmid:8049080 |
| Nauck M et al. | Induction of vascular endothelial growth factor by platelet-activating factor and platelet-derived growth factor is downregulated by corticosteroids. | 1997 | Am. J. Respir. Cell Mol. Biol. | pmid:9115750 |
| Okada S et al. | Transmigration of eosinophils through basement membrane components in vitro: synergistic effects of platelet-activating factor and eosinophil-active cytokines. | 1997 | Am. J. Respir. Cell Mol. Biol. | pmid:9115757 |
| Kroegel C et al. | Modulatory role of protein kinase C on the signal transduction pathway utilized by platelet-activating factor in eosinophil activation. | 1994 | Am. J. Respir. Cell Mol. Biol. | pmid:7946388 |
| Masuda T et al. | Eosinophil penetration through cultured human airway epithelial cell layer. | 1995 | Am. J. Respir. Cell Mol. Biol. | pmid:7766427 |
| Shamsuddin M et al. | Differential regulation of leukotriene and platelet-activating factor synthesis in rat alveolar macrophages. | 1995 | Am. J. Respir. Cell Mol. Biol. | pmid:7766433 |
| Powell WS et al. | 5-oxo-6,8,11,14-eicosatetraenoic acid is a potent stimulator of L-selectin shedding, surface expression of CD11b, actin polymerization, and calcium mobilization in human eosinophils. | 1999 | Am. J. Respir. Cell Mol. Biol. | pmid:9870930 |
| Chase PB et al. | Cloning of a human platelet-activating factor receptor gene: evidence for an intron in the 5'-untranslated region. | 1993 | Am. J. Respir. Cell Mol. Biol. | pmid:8383507 |
| Erger RA and Casale TB | Comparative studies indicate that platelet-activating factor is a relatively weak eosinophilotactic mediator. | 1995 | Am. J. Respir. Cell Mol. Biol. | pmid:7811471 |
| Minamiya Y et al. | Platelet-activating factor mediates intercellular adhesion molecule-1-dependent radical production in the nonhypoxic ischemia rat lung. | 1998 | Am. J. Respir. Cell Mol. Biol. | pmid:9651191 |
| Zoratti EM et al. | Platelet-activating factor primes human eosinophil generation of superoxide. | 1992 | Am. J. Respir. Cell Mol. Biol. | pmid:1309421 |
| Kondo M et al. | Effect of platelet-activating factor on intracellular free calcium in cow tracheal epithelium. | 1994 | Am. J. Respir. Cell Mol. Biol. | pmid:8117446 |
| Neeley SP et al. | Selective regulation of expression of surface adhesion molecules Mac-1, L-selectin, and VLA-4 on human eosinophils and neutrophils. | 1993 | Am. J. Respir. Cell Mol. Biol. | pmid:7686761 |
| Wu T et al. | Endothelin-1 stimulates eicosanoid production in cultured human nasal mucosa. | 1992 | Am. J. Respir. Cell Mol. Biol. | pmid:1311593 |
| Dent G et al. | Protein kinase C inhibition enhances platelet-activating factor-induced eicosanoid production in human eosinophils. | 1998 | Am. J. Respir. Cell Mol. Biol. | pmid:9448055 |
| Elstad MR et al. | Protein kinase C regulates the synthesis of platelet-activating factor by human monocytes. | 1991 | Am. J. Respir. Cell Mol. Biol. | pmid:1846746 |
| Delong P et al. | Bacterial lipopolysaccharide induction of the prostaglandin G/H synthase 2 gene causes thromboxane-dependent pulmonary hypertension in rabbits. | 1999 | Am. J. Respir. Cell Mol. Biol. | pmid:10030848 |
| Zimmerman GA et al. | Juxtacrine intercellular signaling: another way to do it. | 1993 | Am. J. Respir. Cell Mol. Biol. | pmid:7504925 |
| Morland CM et al. | Selective eosinophil leukocyte recruitment by transendothelial migration and not by leukocyte-endothelial cell adhesion. | 1992 | Am. J. Respir. Cell Mol. Biol. | pmid:1316135 |
| Kikuchi I et al. | Eosinophil trans-basement membrane migration induced by interleukin-8 and neutrophils. | 2006 | Am. J. Respir. Cell Mol. Biol. | pmid:16456187 |
| Yukawa T et al. | The effects of activated eosinophils and neutrophils on guinea pig airway epithelium in vitro. | 1990 | Am. J. Respir. Cell Mol. Biol. | pmid:2322467 |
| Gomez J et al. | Characterization of receptors for platelet-activating factor in guinea pig lung membranes. | 1990 | Am. J. Respir. Cell Mol. Biol. | pmid:2167700 |
| Adler KB et al. | Hypersecretion of mucin in response to inflammatory mediators by guinea pig tracheal epithelial cells in vitro is blocked by inhibition of nitric oxide synthase. | 1995 | Am. J. Respir. Cell Mol. Biol. | pmid:7576687 |
| Longphre M and Kleeberger SR | Susceptibility to platelet-activating factor-induced airway hyperreactivity and hyperpermeability: interstrain variation and genetic control. | 1995 | Am. J. Respir. Cell Mol. Biol. | pmid:7576695 |
| Casale TB et al. | Degree of neutrophil chemotaxis is dependent upon the chemoattractant and barrier. | 1992 | Am. J. Respir. Cell Mol. Biol. | pmid:1320901 |
| Miotla JM et al. | Platelet-activating factor plays a pivotal role in the induction of experimental lung injury. | 1998 | Am. J. Respir. Cell Mol. Biol. | pmid:9476906 |