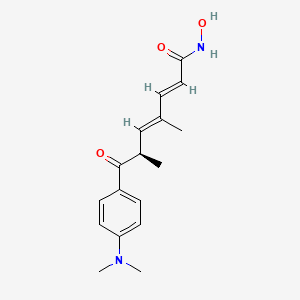
trichostatin A
Trichostatin is a lipid of Polyketides (PK) class. Trichostatin is associated with abnormalities such as Dentatorubral-Pallidoluysian Atrophy, PARAGANGLIOMAS 3, abnormal fragmented structure, Disintegration (morphologic abnormality) and Hyperostosis, Diffuse Idiopathic Skeletal. The involved functions are known as Acetylation, Cell Differentiation process, histone modification, Gene Silencing and Transcriptional Activation. Trichostatin often locates in CD41a, Hematopoietic System, Chromatin Structure, Blood and Endothelium. The associated genes with Trichostatin are SPI1 gene, CELL Gene, Chromatin, CXCR4 gene and DNMT1 gene. The related lipids are Butyrates, Promega, butyrate, Lipopolysaccharides and Steroids. The related experimental models are Knock-out, Mouse Model, Xenograft Model and Cancer Model.
Cross Reference
Introduction
To understand associated biological information of trichostatin A, we collected biological information of abnormalities, associated pathways, cellular/molecular locations, biological functions, related genes/proteins, lipids and common seen animal/experimental models with organized paragraphs from literatures.
What diseases are associated with trichostatin A?
trichostatin A is suspected in Infection, Morphologically altered structure, Ureteral obstruction, Photosensitization, Atherosclerosis, Hypertrophic Cardiomyopathy and other diseases in descending order of the highest number of associated sentences.
Related references are mostly published in these journals:
| Disease | Cross reference | Weighted score | Related literature |
|---|
Possible diseases from mapped MeSH terms on references
We collected disease MeSH terms mapped to the references associated with trichostatin A
PubChem Associated disorders and diseases
What pathways are associated with trichostatin A
Lipid pathways are not clear in current pathway databases. We organized associated pathways with trichostatin A through full-text articles, including metabolic pathways or pathways of biological mechanisms.
Related references are published most in these journals:
| Pathway name | Related literatures |
|---|
PubChem Biomolecular Interactions and Pathways
Link to PubChem Biomolecular Interactions and PathwaysWhat cellular locations are associated with trichostatin A?
Visualization in cellular structure
Associated locations are in red color. Not associated locations are in black.
Related references are published most in these journals:
| Location | Cross reference | Weighted score | Related literatures |
|---|
What functions are associated with trichostatin A?
Related references are published most in these journals:
| Function | Cross reference | Weighted score | Related literatures |
|---|
What lipids are associated with trichostatin A?
Related references are published most in these journals:
| Lipid concept | Cross reference | Weighted score | Related literatures |
|---|
What genes are associated with trichostatin A?
Related references are published most in these journals:
| Gene | Cross reference | Weighted score | Related literatures |
|---|
What common seen animal models are associated with trichostatin A?
Mouse Model
Mouse Model are used in the study 'Regulation of minichromosome maintenance gene family by microRNA-1296 and genistein in prostate cancer.' (Majid S et al., 2010), Mouse Model are used in the study 'Reversal of hypermethylation and reactivation of p16INK4a, RARbeta, and MGMT genes by genistein and other isoflavones from soy.' (Fang MZ et al., 2005) and Mouse Model are used in the study 'Histone deacetylase 3 mediates allergic skin inflammation by regulating expression of MCP1 protein.' (Kim Y et al., 2012).
Xenograft Model
Xenograft Model are used in the study 'Histone deacetylase inhibitors induce growth arrest and differentiation in uveal melanoma.' (Landreville S et al., 2012), Xenograft Model are used in the study 'Extended treatment with physiologic concentrations of dietary phytochemicals results in altered gene expression, reduced growth, and apoptosis of cancer cells.' (Moiseeva EP et al., 2007) and Xenograft Model are used in the study 'Retinoic acid and the histone deacetylase inhibitor trichostatin a inhibit the proliferation of human renal cell carcinoma in a xenograft tumor model.' (Touma SE et al., 2005).
Cancer Model
Cancer Model are used in the study 'Plasma pharmacokinetics and metabolism of the histone deacetylase inhibitor trichostatin a after intraperitoneal administration to mice.' (Sanderson L et al., 2004).
Related references are published most in these journals:
| Model | Cross reference | Weighted score | Related literatures |
|---|
NCBI Entrez Crosslinks
All references with trichostatin A
Download all related citations| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Dogra SC et al. | Evidence that the coactivator CBP/p300 is important for phenobarbital-induced but not basal expression of the CYP2H1 gene. | 2003 | Mol. Pharmacol. | pmid:12488538 |
| Lietz M et al. | RE-1 silencing transcription factor (REST) regulates human synaptophysin gene transcription through an intronic sequence-specific DNA-binding site. | 2003 | Eur. J. Biochem. | pmid:12492469 |
| Yamashita Y et al. | Histone deacetylase inhibitor trichostatin A induces cell-cycle arrest/apoptosis and hepatocyte differentiation in human hepatoma cells. | 2003 | Int. J. Cancer | pmid:12494463 |
| Parekh-Olmedo H et al. | The effect of hydroxyurea and trichostatin a on targeted nucleotide exchange in yeast and Mammalian cells. | 2003 | Ann. N. Y. Acad. Sci. | pmid:14751821 |
| Grisolano JL et al. | An activated receptor tyrosine kinase, TEL/PDGFbetaR, cooperates with AML1/ETO to induce acute myeloid leukemia in mice. | 2003 | Proc. Natl. Acad. Sci. U.S.A. | pmid:12881486 |
| Konduri SD et al. | Promoter methylation and silencing of the tissue factor pathway inhibitor-2 (TFPI-2), a gene encoding an inhibitor of matrix metalloproteinases in human glioma cells. | 2003 | Oncogene | pmid:12881707 |
| Nakajima M et al. | Effects of histone deacetylation and DNA methylation on the constitutive and TCDD-inducible expressions of the human CYP1 family in MCF-7 and HeLa cells. | 2003 | Toxicol. Lett. | pmid:12927368 |
| Papeleu P et al. | Trichostatin A induces differential cell cycle arrests but does not induce apoptosis in primary cultures of mitogen-stimulated rat hepatocytes. | 2003 | J. Hepatol. | pmid:12927923 |
| Senawong T et al. | Involvement of the histone deacetylase SIRT1 in chicken ovalbumin upstream promoter transcription factor (COUP-TF)-interacting protein 2-mediated transcriptional repression. | 2003 | J. Biol. Chem. | pmid:12930829 |
| Mulholland NM et al. | Inhibition of MMTV transcription by HDAC inhibitors occurs independent of changes in chromatin remodeling and increased histone acetylation. | 2003 | Oncogene | pmid:12894222 |
| Banwell CM et al. | Antiproliferative signalling by 1,25(OH)2D3 in prostate and breast cancer is suppressed by a mechanism involving histone deacetylation. | 2003 | Recent Results Cancer Res. | pmid:12899515 |
| Yan Y et al. | Analysis of specific lysine histone H3 and H4 acetylation and methylation status in clones of cells with a gene silenced by nickel exposure. | 2003 | Toxicol. Appl. Pharmacol. | pmid:12902198 |
| Augenlicht L et al. | Repression of MUC2 gene expression by butyrate, a physiological regulator of intestinal cell maturation. | 2003 | Oncogene | pmid:12902981 |
| Cho B et al. | Promoter hypomethylation of a novel cancer/testis antigen gene CAGE is correlated with its aberrant expression and is seen in premalignant stage of gastric carcinoma. | 2003 | Biochem. Biophys. Res. Commun. | pmid:12849980 |
| Thomas RM et al. | Regulation of mouse mammary tumor virus env transcriptional activator initiated mammary tumor virus superantigen transcripts in lymphomas of SJL/J mice: role of Ikaros, demethylation, and chromatin structural change in the transcriptional activation of mammary tumor virus superantigen. | 2003 | J. Immunol. | pmid:12496403 |
| Xu Y et al. | SHP-1 sensitizes MCF-7 cells to trichostatin A-induced apoptosis by modulating PI3K-dependent events. | 2003 | Cell Death Differ. | pmid:14502244 |
| Donadelli M et al. | Trichostatin A, an inhibitor of histone deacetylases, strongly suppresses growth of pancreatic adenocarcinoma cells. | 2003 | Mol. Carcinog. | pmid:14502645 |
| Klisovic DD et al. | Depsipeptide (FR901228) inhibits proliferation and induces apoptosis in primary and metastatic human uveal melanoma cell lines. | 2003 | Invest. Ophthalmol. Vis. Sci. | pmid:12766035 |
| Génin P et al. | Impairment of interferon-induced IRF-7 gene expression due to inhibition of ISGF3 formation by trichostatin A. | 2003 | J. Virol. | pmid:12768031 |
| Bartoli A et al. | Effect of trichostatin a and 5'-azacytidine on transgene reactivation in U937 transduced cells. | 2003 | Pharmacol. Res. | pmid:12770523 |
| Koeller KM et al. | Chemical genetic modifier screens: small molecule trichostatin suppressors as probes of intracellular histone and tubulin acetylation. | 2003 | Chem. Biol. | pmid:12770822 |
| Agoulnik IU et al. | Repressors of androgen and progesterone receptor action. | 2003 | J. Biol. Chem. | pmid:12771131 |
| Huang Y et al. | Hypermethylation, but not LOH, is associated with the low expression of MT1G and CRABP1 in papillary thyroid carcinoma. | 2003 | Int. J. Cancer | pmid:12640681 |
| Méndez-Pertuz M et al. | The thyroid hormone receptor antagonizes CREB-mediated transcription. | 2003 | EMBO J. | pmid:12805224 |
| Wong JE et al. | Therapeutic strategies in gastric cancer. | 2003 | J. Clin. Oncol. | pmid:14645406 |
| Suzuki M et al. | Direct association between PU.1 and MeCP2 that recruits mSin3A-HDAC complex for PU.1-mediated transcriptional repression. | 2003 | Oncogene | pmid:14647463 |
| Kim HS et al. | Regulation of the tyrosine hydroxylase gene promoter by histone deacetylase inhibitors. | 2003 | Biochem. Biophys. Res. Commun. | pmid:14651963 |
| Coombes MM et al. | Resetting the histone code at CDKN2A in HNSCC by inhibition of DNA methylation. | 2003 | Oncogene | pmid:14654786 |
| Chan AS et al. | Downregulation of ID4 by promoter hypermethylation in gastric adenocarcinoma. | 2003 | Oncogene | pmid:14534543 |
| Allison SJ and Milner J | Loss of p53 has site-specific effects on histone H3 modification, including serine 10 phosphorylation important for maintenance of ploidy. | 2003 | Cancer Res. | pmid:14583461 |
| Mai A et al. | Discovery of (aryloxopropenyl)pyrrolyl hydroxyamides as selective inhibitors of class IIa histone deacetylase homologue HD1-A. | 2003 | J. Med. Chem. | pmid:14584932 |
| Ju R and Muller MT | Histone deacetylase inhibitors activate p21(WAF1) expression via ATM. | 2003 | Cancer Res. | pmid:12782595 |
| Chua YL et al. | The transcriptional enhancer of the pea plastocyanin gene associates with the nuclear matrix and regulates gene expression through histone acetylation. | 2003 | Plant Cell | pmid:12782737 |
| Cecconi D et al. | Proteomic profiling of pancreatic ductal carcinoma cell lines treated with trichostatin-A. | 2003 | Electrophoresis | pmid:12783462 |
| Fukuoka M et al. | Negative regulation of forkhead transcription factor AFX (Foxo4) by CBP-induced acetylation. | 2003 | Int. J. Mol. Med. | pmid:12964026 |
| Vanommeslaeghe K et al. | Ab initio study of the binding of Trichostatin A (TSA) in the active site of histone deacetylase like protein (HDLP). | 2003 | Org. Biomol. Chem. | pmid:12968347 |
| Huang H et al. | [HDAC1 expression and effect of TSA on proliferation and apoptosis of A549 cells]. | 2003 | Ai Zheng | pmid:12969522 |
| Ohike N et al. | Clinicopathological significance and molecular regulation of maspin expression in ductal adenocarcinoma of the pancreas. | 2003 | Cancer Lett. | pmid:12969792 |
| Fass DM et al. | Deacetylase activity is required for cAMP activation of a subset of CREB target genes. | 2003 | J. Biol. Chem. | pmid:12939274 |
| Zhao C et al. | A composite motif of the Drosophila morphogenetic protein bicoid critical to transcription control. | 2003 | J. Biol. Chem. | pmid:12939280 |
| Hinoi T et al. | Silencing of CDX2 expression in colon cancer via a dominant repression pathway. | 2003 | J. Biol. Chem. | pmid:12947088 |
| Park WH et al. | Trichostatin inhibits the growth of ACHN renal cell carcinoma cells via cell cycle arrest in association with p27, or apoptosis. | 2003 | Int. J. Oncol. | pmid:12684681 |
| Farhan H et al. | Genistein inhibits vitamin D hydroxylases CYP24 and CYP27B1 expression in prostate cells. | 2003 | J. Steroid Biochem. Mol. Biol. | pmid:12732287 |
| Chambers AE et al. | Histone acetylation-mediated regulation of genes in leukaemic cells. | 2003 | Eur. J. Cancer | pmid:12736119 |
| Hoever G et al. | The mechanism of 3'-azido-2',3'-dideoxythymidine resistance to human lymphoid cells. | 2003 | Int. J. Mol. Med. | pmid:12736716 |
| Valenta T et al. | HMG box transcription factor TCF-4's interaction with CtBP1 controls the expression of the Wnt target Axin2/Conductin in human embryonic kidney cells. | 2003 | Nucleic Acids Res. | pmid:12711682 |
| Arora T et al. | PIASx is a transcriptional co-repressor of signal transducer and activator of transcription 4. | 2003 | J. Biol. Chem. | pmid:12716907 |
| Sonoyama K et al. | Upregulation of activin A gene by butyrate in human colon cancer cell lines. | 2003 | Am. J. Physiol. Gastrointest. Liver Physiol. | pmid:12540370 |
| Kagoshima M et al. | Glucocorticoid suppression of nuclear factor-kappa B: a role for histone modifications. | 2003 | Biochem. Soc. Trans. | pmid:12546654 |
| Bhat RA et al. | Alteration of GCN5 levels in maize reveals dynamic responses to manipulating histone acetylation. | 2003 | Plant J. | pmid:12581304 |