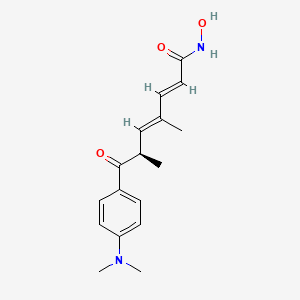
trichostatin A
Trichostatin is a lipid of Polyketides (PK) class. Trichostatin is associated with abnormalities such as Dentatorubral-Pallidoluysian Atrophy, PARAGANGLIOMAS 3, abnormal fragmented structure, Disintegration (morphologic abnormality) and Hyperostosis, Diffuse Idiopathic Skeletal. The involved functions are known as Acetylation, Cell Differentiation process, histone modification, Gene Silencing and Transcriptional Activation. Trichostatin often locates in CD41a, Hematopoietic System, Chromatin Structure, Blood and Endothelium. The associated genes with Trichostatin are SPI1 gene, CELL Gene, Chromatin, CXCR4 gene and DNMT1 gene. The related lipids are Butyrates, Promega, butyrate, Lipopolysaccharides and Steroids. The related experimental models are Knock-out, Mouse Model, Xenograft Model and Cancer Model.
Cross Reference
Introduction
To understand associated biological information of trichostatin A, we collected biological information of abnormalities, associated pathways, cellular/molecular locations, biological functions, related genes/proteins, lipids and common seen animal/experimental models with organized paragraphs from literatures.
What diseases are associated with trichostatin A?
trichostatin A is suspected in Infection, Morphologically altered structure, Ureteral obstruction, Photosensitization, Atherosclerosis, Hypertrophic Cardiomyopathy and other diseases in descending order of the highest number of associated sentences.
Related references are mostly published in these journals:
| Disease | Cross reference | Weighted score | Related literature |
|---|
Possible diseases from mapped MeSH terms on references
We collected disease MeSH terms mapped to the references associated with trichostatin A
PubChem Associated disorders and diseases
What pathways are associated with trichostatin A
Lipid pathways are not clear in current pathway databases. We organized associated pathways with trichostatin A through full-text articles, including metabolic pathways or pathways of biological mechanisms.
Related references are published most in these journals:
| Pathway name | Related literatures |
|---|
PubChem Biomolecular Interactions and Pathways
Link to PubChem Biomolecular Interactions and PathwaysWhat cellular locations are associated with trichostatin A?
Visualization in cellular structure
Associated locations are in red color. Not associated locations are in black.
Related references are published most in these journals:
| Location | Cross reference | Weighted score | Related literatures |
|---|
What functions are associated with trichostatin A?
Related references are published most in these journals:
| Function | Cross reference | Weighted score | Related literatures |
|---|
What lipids are associated with trichostatin A?
Related references are published most in these journals:
| Lipid concept | Cross reference | Weighted score | Related literatures |
|---|
What genes are associated with trichostatin A?
Related references are published most in these journals:
| Gene | Cross reference | Weighted score | Related literatures |
|---|
What common seen animal models are associated with trichostatin A?
Mouse Model
Mouse Model are used in the study 'Regulation of minichromosome maintenance gene family by microRNA-1296 and genistein in prostate cancer.' (Majid S et al., 2010), Mouse Model are used in the study 'Reversal of hypermethylation and reactivation of p16INK4a, RARbeta, and MGMT genes by genistein and other isoflavones from soy.' (Fang MZ et al., 2005) and Mouse Model are used in the study 'Histone deacetylase 3 mediates allergic skin inflammation by regulating expression of MCP1 protein.' (Kim Y et al., 2012).
Xenograft Model
Xenograft Model are used in the study 'Histone deacetylase inhibitors induce growth arrest and differentiation in uveal melanoma.' (Landreville S et al., 2012), Xenograft Model are used in the study 'Extended treatment with physiologic concentrations of dietary phytochemicals results in altered gene expression, reduced growth, and apoptosis of cancer cells.' (Moiseeva EP et al., 2007) and Xenograft Model are used in the study 'Retinoic acid and the histone deacetylase inhibitor trichostatin a inhibit the proliferation of human renal cell carcinoma in a xenograft tumor model.' (Touma SE et al., 2005).
Cancer Model
Cancer Model are used in the study 'Plasma pharmacokinetics and metabolism of the histone deacetylase inhibitor trichostatin a after intraperitoneal administration to mice.' (Sanderson L et al., 2004).
Related references are published most in these journals:
| Model | Cross reference | Weighted score | Related literatures |
|---|
NCBI Entrez Crosslinks
All references with trichostatin A
Download all related citations| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Bence M et al. | Transcriptional modulation of monoaminergic neurotransmission genes by the histone deacetylase inhibitor trichostatin A in neuroblastoma cells. | 2012 | J Neural Transm | pmid:21785940 |
| Suh HS et al. | Histone deacetylase inhibitors suppress the expression of inflammatory and innate immune response genes in human microglia and astrocytes. | 2010 | J Neuroimmune Pharmacol | pmid:20157787 |
| Fleiss B et al. | Neuroprotection by the histone deacetylase inhibitor trichostatin A in a model of lipopolysaccharide-sensitised neonatal hypoxic-ischaemic brain injury. | 2012 | J Neuroinflammation | pmid:22512781 |
| Noro R et al. | The anticancer effect of histone deacetylase inhibitors and combination with the cytotoxic agents in lung cancer cells: biological analyses for future clinical application. | 2009 | J Nippon Med Sch | pmid:19305113 |
| Por ED et al. | Trichostatin A Inhibits Retinal Pigmented Epithelium Activation in an In Vitro Model of Proliferative Vitreoretinopathy. | 2016 | J Ocul Pharmacol Ther | pmid:27494828 |
| Sun G et al. | Insights into the lysine acetylproteome of human sperm. | 2014 | J Proteomics | pmid:25038526 |
| Gavin DP et al. | Histone deacetylase inhibitors and candidate gene expression: An in vivo and in vitro approach to studying chromatin remodeling in a clinical population. | 2009 | J Psychiatr Res | pmid:19187942 |
| Gavin DP et al. | Dimethylated lysine 9 of histone 3 is elevated in schizophrenia and exhibits a divergent response to histone deacetylase inhibitors in lymphocyte cultures. | 2009 | J Psychiatry Neurosci | pmid:19448855 |
| Albertus DL et al. | AZGP1 autoantibody predicts survival and histone deacetylase inhibitors increase expression in lung adenocarcinoma. | 2008 | J Thorac Oncol | pmid:18978557 |
| Seo SK et al. | Histone deacetylase inhibitors sensitize human non-small cell lung cancer cells to ionizing radiation through acetyl p53-mediated c-myc down-regulation. | 2011 | J Thorac Oncol | pmid:21642861 |
| Selinger CI et al. | Loss of special AT-rich binding protein 1 expression is a marker of poor survival in lung cancer. | 2011 | J Thorac Oncol | pmid:21597389 |
| Kakihana M et al. | Induction of E-cadherin in lung cancer and interaction with growth suppression by histone deacetylase inhibition. | 2009 | J Thorac Oncol | pmid:20009910 |
| Kimura T et al. | Chromium (VI) inhibits mouse metallothionein-I gene transcription by modifying the transcription potential of the co-activator p300. | 2011 | J Toxicol Sci | pmid:21467744 |
| Simões-Pires CA et al. | Simultaneous Measurement of HDAC1 and HDAC6 Activity in HeLa Cells Using UHPLC-MS. | 2017 | J Vis Exp | pmid:28829415 |
| Azuma R et al. | Combinational Treatment of Trichostatin A and Vitamin C Improves the Efficiency of Cloning Mice by Somatic Cell Nuclear Transfer. | 2018 | J Vis Exp | pmid:29757287 |
| Valapour M et al. | Histone deacetylation inhibits IL4 gene expression in T cells. | 2002 | J. Allergy Clin. Immunol. | pmid:11842291 |
| Su RC et al. | Epigenetic regulation of established human type 1 versus type 2 cytokine responses. | 2008 | J. Allergy Clin. Immunol. | pmid:17980413 |
| Milagre I et al. | Chromatin-modifying agents increase transcription of CYP46A1, a key player in brain cholesterol elimination. | 2010 | J. Alzheimers Dis. | pmid:20930312 |
| Kudo K et al. | Biosynthetic Origin of the Hydroxamic Acid Moiety of Trichostatin A: Identification of Unprecedented Enzymatic Machinery Involved in Hydroxylamine Transfer. | 2017 | J. Am. Chem. Soc. | pmid:28497964 |
| Ren C et al. | Peptide mass mapping of acetylated isoforms of histone H4 from mouse lymphosarcoma cells treated with histone deacetylase (HDACs) inhibitors. | 2005 | J. Am. Soc. Mass Spectrom. | pmid:16099169 |
| Yoshikawa M et al. | Inhibition of histone deacetylase activity suppresses epithelial-to-mesenchymal transition induced by TGF-beta1 in human renal epithelial cells. | 2007 | J. Am. Soc. Nephrol. | pmid:17135397 |
| Yu Z et al. | Histone deacetylases augment cytokine induction of the iNOS gene. | 2002 | J. Am. Soc. Nephrol. | pmid:12138131 |
| Hou W et al. | Hypoxia-induced deacetylation is required for tetraploid differentiation in response to testicular ischemia-reperfusion (IR) injury. | 2012 Nov-Dec | J. Androl. | pmid:22556385 |
| Fenic I et al. | In vivo application of histone deacetylase inhibitor trichostatin-a impairs murine male meiosis. | 2008 Mar-Apr | J. Androl. | pmid:18046049 |
| Fenic I et al. | In vivo effects of histone-deacetylase inhibitor trichostatin-A on murine spermatogenesis. | 2004 Sep-Oct | J. Androl. | pmid:15292114 |
| Shi LH et al. | Trichostatin A and nuclear reprogramming of cloned rabbit embryos. | 2008 | J. Anim. Sci. | pmid:18245503 |
| Ueda JY et al. | JBIR-17, a novel trichostatin analog from Streptomyces sp. 26634. | 2009 | J. Antibiot. | pmid:19300468 |
| Yoshida M et al. | Structural specificity for biological activity of trichostatin A, a specific inhibitor of mammalian cell cycle with potent differentiation-inducing activity in Friend leukemia cells. | 1990 | J. Antibiot. | pmid:2211374 |
| Otoguro K et al. | Screening for new antitrichomonal substances of microbial origin and antitrichomonal activity of trichostatin A. | 1988 | J. Antibiot. | pmid:3372352 |
| Takahashi I et al. | Selective inhibition of IL-2 gene expression by trichostatin A, a potent inhibitor of mammalian histone deacetylase. | 1996 | J. Antibiot. | pmid:8682722 |
| Komatsu Y and Hayashi H | Histone deacetylase inhibitors up-regulate the expression of cell surface MHC class-I molecules in B16/BL6 cells. | 1998 | J. Antibiot. | pmid:9531994 |
| Koguchi Y et al. | Trichostatin A and herboxidiene up-regulate the gene expression of low density lipoprotein receptor. | 1997 | J. Antibiot. | pmid:9592573 |
| Kim YB et al. | Mechanism of cell cycle arrest caused by histone deacetylase inhibitors in human carcinoma cells. | 2000 | J. Antibiot. | pmid:11132966 |
| Dupré-Aucouturier S et al. | Trichostatin A, a histone deacetylase inhibitor, modulates unloaded-induced skeletal muscle atrophy. | 2015 | J. Appl. Physiol. | pmid:26112243 |
| Jafari S et al. | Epigenetic modification does not determine the time of POU5F1 transcription activation in cloned bovine embryos. | 2011 | J. Assist. Reprod. Genet. | pmid:22020531 |
| Slattery EL et al. | Epigenetic influences on sensory regeneration: histone deacetylases regulate supporting cell proliferation in the avian utricle. | 2009 | J. Assoc. Res. Otolaryngol. | pmid:19340485 |
| Azechi T et al. | Trichostatin A, an HDAC class I/II inhibitor, promotes Pi-induced vascular calcification via up-regulation of the expression of alkaline phosphatase. | 2013 | J. Atheroscler. Thromb. | pmid:23518467 |
| Okamoto H et al. | Trichostatin A, an inhibitor of histone deacetylase, inhibits smooth muscle cell proliferation via induction of p21(WAF1). | 2006 | J. Atheroscler. Thromb. | pmid:16908950 |
| Reilly CM et al. | The histone deacetylase inhibitor trichostatin A upregulates regulatory T cells and modulates autoimmunity in NZB/W F1 mice. | 2008 | J. Autoimmun. | pmid:18650065 |
| Hildmann C et al. | A new amidohydrolase from Bordetella or Alcaligenes strain FB188 with similarities to histone deacetylases. | 2004 | J. Bacteriol. | pmid:15060035 |
| Maruoka H et al. | Low-molecular-weight compounds having neurotrophic activity in cultured PC12 cells and neurons. | 2011 | J. Biochem. | pmid:21908547 |
| Yamamoto I et al. | Histone hyperacetylation plays a role in augmentation of IL-4-induced IgE production in LPS-stimulated murine B-lymphocytes by sodium butyrate. | 1996 | J. Biochem. | pmid:8827437 |
| Zhao W et al. | The essential role of histone H3 Lys9 di-methylation and MeCP2 binding in MGMT silencing with poor DNA methylation of the promoter CpG island. | 2005 | J. Biochem. | pmid:15809347 |
| Kook SH et al. | Epstein-Barr virus-infected Akata cells are sensitive to histone deacetylase inhibitor TSA-provoked apoptosis. | 2005 | J. Biochem. Mol. Biol. | pmid:16336792 |
| Zhang Y and Zhang B | Trichostatin A, an Inhibitor of Histone Deacetylase, Inhibits the Viability and Invasiveness of Hypoxic Rheumatoid Arthritis Fibroblast-Like Synoviocytes via PI3K/Akt Signaling. | 2016 | J. Biochem. Mol. Toxicol. | pmid:26509796 |
| Jin B and Ryu DY | Regulation of CYP1A2 by histone deacetylase inhibitors in mouse hepatocytes. | 2004 | J. Biochem. Mol. Toxicol. | pmid:15252868 |
| Sanyal S et al. | Differential regulation of the orphan nuclear receptor small heterodimer partner (SHP) gene promoter by orphan nuclear receptor ERR isoforms. | 2002 | J. Biol. Chem. | pmid:11705994 |
| Galasinski SC et al. | Global regulation of post-translational modifications on core histones. | 2002 | J. Biol. Chem. | pmid:11709551 |
| Yin L et al. | Butyrate suppression of colonocyte NF-kappa B activation and cellular proteasome activity. | 2001 | J. Biol. Chem. | pmid:11572859 |
| Liou JS et al. | Oncogenic ras mediates apoptosis in response to protein kinase C inhibition through the generation of reactive oxygen species. | 2000 | J. Biol. Chem. | pmid:10967125 |
| Sakai N et al. | Involvement of histone acetylation in ovarian steroid-induced decidualization of human endometrial stromal cells. | 2003 | J. Biol. Chem. | pmid:12609987 |
| Kurtev V et al. | Transcriptional regulation by the repressor of estrogen receptor activity via recruitment of histone deacetylases. | 2004 | J. Biol. Chem. | pmid:15140878 |
| Klampfer L et al. | Requirement of histone deacetylase activity for signaling by STAT1. | 2004 | J. Biol. Chem. | pmid:15123634 |
| Fu M et al. | The androgen receptor acetylation site regulates cAMP and AKT but not ERK-induced activity. | 2004 | J. Biol. Chem. | pmid:15123687 |
| Rigamonti D et al. | Loss of huntingtin function complemented by small molecules acting as repressor element 1/neuron restrictive silencer element silencer modulators. | 2007 | J. Biol. Chem. | pmid:17565993 |
| Kuno A et al. | Resveratrol improves cardiomyopathy in dystrophin-deficient mice through SIRT1 protein-mediated modulation of p300 protein. | 2013 | J. Biol. Chem. | pmid:23297412 |
| Phiel CJ et al. | Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. | 2001 | J. Biol. Chem. | pmid:11473107 |
| Jacob AL et al. | Acetylation of steroidogenic factor 1 protein regulates its transcriptional activity and recruits the coactivator GCN5. | 2001 | J. Biol. Chem. | pmid:11479297 |
| Gregory RI et al. | Inhibition of histone deacetylases alters allelic chromatin conformation at the imprinted U2af1-rs1 locus in mouse embryonic stem cells. | 2002 | J. Biol. Chem. | pmid:11821379 |
| Lim Y et al. | Trichostatin A-induced detransformation correlates with decreased focal adhesion kinase phosphorylation at tyrosine 861 in ras-transformed fibroblasts. | 2002 | J. Biol. Chem. | pmid:11821402 |
| Wilson MA et al. | The histone deacetylase inhibitor trichostatin A blocks progesterone receptor-mediated transactivation of the mouse mammary tumor virus promoter in vivo. | 2002 | J. Biol. Chem. | pmid:11821430 |
| Hattori N et al. | Epigenetic control of mouse Oct-4 gene expression in embryonic stem cells and trophoblast stem cells. | 2004 | J. Biol. Chem. | pmid:14761969 |
| Christian M et al. | Characterization of four autonomous repression domains in the corepressor receptor interacting protein 140. | 2004 | J. Biol. Chem. | pmid:14736873 |
| Bu Y and Gelman IH | v-Src-mediated down-regulation of SSeCKS metastasis suppressor gene promoter by the recruitment of HDAC1 into a USF1-Sp1-Sp3 complex. | 2007 | J. Biol. Chem. | pmid:17626016 |
| Singal R et al. | Methylation of promoter proximal-transcribed sequences of an embryonic globin gene inhibits transcription in primary erythroid cells and promotes formation of a cell type-specific methyl cytosine binding complex. | 2002 | J. Biol. Chem. | pmid:11684679 |
| Ammanamanchi S et al. | Acetylated sp3 is a transcriptional activator. | 2003 | J. Biol. Chem. | pmid:12837748 |
| Shan J et al. | ATF4-dependent regulation of the JMJD3 gene during amino acid deprivation can be rescued in Atf4-deficient cells by inhibition of deacetylation. | 2012 | J. Biol. Chem. | pmid:22955275 |
| Wang J et al. | Histone deacetylase inhibitors suppress TF-kappaB-dependent agonist-driven tissue factor expression in endothelial cells and monocytes. | 2007 | J. Biol. Chem. | pmid:17675290 |
| Hishiki T et al. | BCL3 acts as a negative regulator of transcription from the human T-cell leukemia virus type 1 long terminal repeat through interactions with TORC3. | 2007 | J. Biol. Chem. | pmid:17644518 |
| Lin AC et al. | The N termini of Friend of GATA (FOG) proteins define a novel transcriptional repression motif and a superfamily of transcriptional repressors. | 2004 | J. Biol. Chem. | pmid:15507435 |
| Chang S and Pikaard CS | Transcript profiling in Arabidopsis reveals complex responses to global inhibition of DNA methylation and histone deacetylation. | 2005 | J. Biol. Chem. | pmid:15516340 |
| Wang S and Zhu J | The hTERT gene is embedded in a nuclease-resistant chromatin domain. | 2004 | J. Biol. Chem. | pmid:15516693 |
| Diamond SE and Gutierrez-Hartmann A | The Pit-1beta domain dictates active repression and alteration of histone acetylation of the proximal prolactin promoter. | 2000 | J. Biol. Chem. | pmid:10921928 |
| Spallotta F et al. | A nitric oxide-dependent cross-talk between class I and III histone deacetylases accelerates skin repair. | 2013 | J. Biol. Chem. | pmid:23463510 |
| Mogal AP et al. | Haploinsufficient prostate tumor suppression by Nkx3.1: a role for chromatin accessibility in dosage-sensitive gene regulation. | 2007 | J. Biol. Chem. | pmid:17602165 |
| Wong CY et al. | Epigenetic regulation of phosphatidylinositol 3,4,5-triphosphate-dependent Rac exchanger 1 gene expression in prostate cancer cells. | 2011 | J. Biol. Chem. | pmid:21636851 |
| Carmen AA et al. | HDA1 and HDA3 are components of a yeast histone deacetylase (HDA) complex. | 1996 | J. Biol. Chem. | pmid:8663039 |
| Asoh S et al. | The super anti-apoptotic factor Bcl-xFNK constructed by disturbing intramolecular polar interactions in rat Bcl-xL. | 2000 | J. Biol. Chem. | pmid:10970895 |
| Cong YS and Bacchetti S | Histone deacetylation is involved in the transcriptional repression of hTERT in normal human cells. | 2000 | J. Biol. Chem. | pmid:10986277 |
| Su M et al. | Recruitment of nuclear factor Y to the inverted CCAAT element (ICE) by c-Jun and E1A stimulates basal transcription of the bone sialoprotein gene in osteosarcoma cells. | 2005 | J. Biol. Chem. | pmid:16087680 |
| Milutinovic S et al. | Proliferating cell nuclear antigen associates with histone deacetylase activity, integrating DNA replication and chromatin modification. | 2002 | J. Biol. Chem. | pmid:11929879 |
| Kijima M et al. | Trapoxin, an antitumor cyclic tetrapeptide, is an irreversible inhibitor of mammalian histone deacetylase. | 1993 | J. Biol. Chem. | pmid:8226751 |
| Waterborg JH | Dynamics of histone acetylation in Chlamydomonas reinhardtii. | 1998 | J. Biol. Chem. | pmid:9765294 |
| Simboeck E et al. | A phosphorylation switch regulates the transcriptional activation of cell cycle regulator p21 by histone deacetylase inhibitors. | 2010 | J. Biol. Chem. | pmid:20952396 |
| Yamaguchi K et al. | Histone deacetylase inhibitors suppress the induction of c-Jun and its target genes including COX-2. | 2005 | J. Biol. Chem. | pmid:15994313 |
| Wooten LG and Ogretmen B | Sp1/Sp3-dependent regulation of human telomerase reverse transcriptase promoter activity by the bioactive sphingolipid ceramide. | 2005 | J. Biol. Chem. | pmid:15951564 |
| Marks J et al. | Two functionally divergent p53-responsive elements in the rat bradykinin B2 receptor promoter. | 2003 | J. Biol. Chem. | pmid:12791684 |
| Asthana J et al. | Inhibition of HDAC6 deacetylase activity increases its binding with microtubules and suppresses microtubule dynamic instability in MCF-7 cells. | 2013 | J. Biol. Chem. | pmid:23798680 |
| Liao M et al. | Coactivator function of positive cofactor 4 (PC4) in Sp1-directed luteinizing hormone receptor (LHR) gene transcription. | 2011 | J. Biol. Chem. | pmid:21193408 |
| Hayakawa K et al. | Epigenetic switching by the metabolism-sensing factors in the generation of orexin neurons from mouse embryonic stem cells. | 2013 | J. Biol. Chem. | pmid:23625921 |
| Liu CJ et al. | Leukemia/lymphoma-related factor, a POZ domain-containing transcriptional repressor, interacts with histone deacetylase-1 and inhibits cartilage oligomeric matrix protein gene expression and chondrogenesis. | 2004 | J. Biol. Chem. | pmid:15337766 |
| Muralidhar SA et al. | Histone deacetylase 9 activates gamma-globin gene expression in primary erythroid cells. | 2011 | J. Biol. Chem. | pmid:21078662 |
| Hayakawa F et al. | Acetylation of PML is involved in histone deacetylase inhibitor-mediated apoptosis. | 2008 | J. Biol. Chem. | pmid:18621739 |
| Kawamura T et al. | Acetylation of GATA-4 is involved in the differentiation of embryonic stem cells into cardiac myocytes. | 2005 | J. Biol. Chem. | pmid:15764815 |
| Hichino A et al. | Down-regulation of Claudin-2 Expression and Proliferation by Epigenetic Inhibitors in Human Lung Adenocarcinoma A549 Cells. | 2017 | J. Biol. Chem. | pmid:28057758 |
| Johnson CA et al. | Deacetylase activity associates with topoisomerase II and is necessary for etoposide-induced apoptosis. | 2001 | J. Biol. Chem. | pmid:11136718 |
| Walia H et al. | Histone acetylation is required to maintain the unfolded nucleosome structure associated with transcribing DNA. | 1998 | J. Biol. Chem. | pmid:9603965 |
| Yoshioka H et al. | Nonsteroidal anti-inflammatory drug-activated gene (NAG-1/GDF15) expression is increased by the histone deacetylase inhibitor trichostatin A. | 2008 | J. Biol. Chem. | pmid:18801729 |
| Sakamoto S et al. | Histone deacetylase activity is required to recruit RNA polymerase II to the promoters of selected interferon-stimulated early response genes. | 2004 | J. Biol. Chem. | pmid:15194680 |
| Ng Y et al. | Expression of the human myotonic dystrophy kinase-related Cdc42-binding kinase gamma is regulated by promoter DNA methylation and Sp1 binding. | 2004 | J. Biol. Chem. | pmid:15194684 |