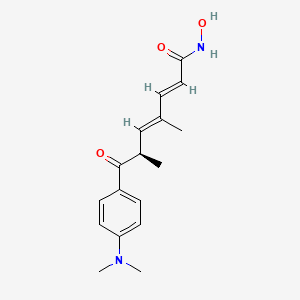
trichostatin A
Trichostatin is a lipid of Polyketides (PK) class. Trichostatin is associated with abnormalities such as Dentatorubral-Pallidoluysian Atrophy, PARAGANGLIOMAS 3, abnormal fragmented structure, Disintegration (morphologic abnormality) and Hyperostosis, Diffuse Idiopathic Skeletal. The involved functions are known as Acetylation, Cell Differentiation process, histone modification, Gene Silencing and Transcriptional Activation. Trichostatin often locates in CD41a, Hematopoietic System, Chromatin Structure, Blood and Endothelium. The associated genes with Trichostatin are SPI1 gene, CELL Gene, Chromatin, CXCR4 gene and DNMT1 gene. The related lipids are Butyrates, Promega, butyrate, Lipopolysaccharides and Steroids. The related experimental models are Knock-out, Mouse Model, Xenograft Model and Cancer Model.
Cross Reference
Introduction
To understand associated biological information of trichostatin A, we collected biological information of abnormalities, associated pathways, cellular/molecular locations, biological functions, related genes/proteins, lipids and common seen animal/experimental models with organized paragraphs from literatures.
What diseases are associated with trichostatin A?
trichostatin A is suspected in Infection, Morphologically altered structure, Ureteral obstruction, Photosensitization, Atherosclerosis, Hypertrophic Cardiomyopathy and other diseases in descending order of the highest number of associated sentences.
Related references are mostly published in these journals:
| Disease | Cross reference | Weighted score | Related literature |
|---|
Possible diseases from mapped MeSH terms on references
We collected disease MeSH terms mapped to the references associated with trichostatin A
PubChem Associated disorders and diseases
What pathways are associated with trichostatin A
Lipid pathways are not clear in current pathway databases. We organized associated pathways with trichostatin A through full-text articles, including metabolic pathways or pathways of biological mechanisms.
Related references are published most in these journals:
| Pathway name | Related literatures |
|---|
PubChem Biomolecular Interactions and Pathways
Link to PubChem Biomolecular Interactions and PathwaysWhat cellular locations are associated with trichostatin A?
Visualization in cellular structure
Associated locations are in red color. Not associated locations are in black.
Related references are published most in these journals:
| Location | Cross reference | Weighted score | Related literatures |
|---|
What functions are associated with trichostatin A?
Related references are published most in these journals:
| Function | Cross reference | Weighted score | Related literatures |
|---|
What lipids are associated with trichostatin A?
Related references are published most in these journals:
| Lipid concept | Cross reference | Weighted score | Related literatures |
|---|
What genes are associated with trichostatin A?
Related references are published most in these journals:
| Gene | Cross reference | Weighted score | Related literatures |
|---|
What common seen animal models are associated with trichostatin A?
Mouse Model
Mouse Model are used in the study 'Regulation of minichromosome maintenance gene family by microRNA-1296 and genistein in prostate cancer.' (Majid S et al., 2010), Mouse Model are used in the study 'Reversal of hypermethylation and reactivation of p16INK4a, RARbeta, and MGMT genes by genistein and other isoflavones from soy.' (Fang MZ et al., 2005) and Mouse Model are used in the study 'Histone deacetylase 3 mediates allergic skin inflammation by regulating expression of MCP1 protein.' (Kim Y et al., 2012).
Xenograft Model
Xenograft Model are used in the study 'Histone deacetylase inhibitors induce growth arrest and differentiation in uveal melanoma.' (Landreville S et al., 2012), Xenograft Model are used in the study 'Extended treatment with physiologic concentrations of dietary phytochemicals results in altered gene expression, reduced growth, and apoptosis of cancer cells.' (Moiseeva EP et al., 2007) and Xenograft Model are used in the study 'Retinoic acid and the histone deacetylase inhibitor trichostatin a inhibit the proliferation of human renal cell carcinoma in a xenograft tumor model.' (Touma SE et al., 2005).
Cancer Model
Cancer Model are used in the study 'Plasma pharmacokinetics and metabolism of the histone deacetylase inhibitor trichostatin a after intraperitoneal administration to mice.' (Sanderson L et al., 2004).
Related references are published most in these journals:
| Model | Cross reference | Weighted score | Related literatures |
|---|
NCBI Entrez Crosslinks
All references with trichostatin A
Download all related citations| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Ou JN et al. | Histone deacetylase inhibitor Trichostatin A induces global and gene-specific DNA demethylation in human cancer cell lines. | 2007 | Biochem. Pharmacol. | pmid:17276411 |
| McBain JA et al. | Apoptotic death in adenocarcinoma cell lines induced by butyrate and other histone deacetylase inhibitors. | 1997 | Biochem. Pharmacol. | pmid:9214697 |
| Mikkelsen IM et al. | Activation of the gamma-glutamyltransferase promoter 2 in the rat colon carcinoma cell line CC531 by histone deacetylase inhibitors is mediated through the Sp1 binding motif. | 2002 | Biochem. Pharmacol. | pmid:12123752 |
| Kohge T et al. | Promotion of antigen-specific antibody production in murine B cells by a moderate increase in histone acetylation. | 1998 | Biochem. Pharmacol. | pmid:9825735 |
| Jalonen U et al. | Inhibition of tristetraprolin expression by dexamethasone in activated macrophages. | 2005 | Biochem. Pharmacol. | pmid:15710351 |
| Brieger A et al. | In bcr-abl-positive myeloid cells resistant to conventional chemotherapeutic agents, expression of Par-4 increases sensitivity to imatinib (STI571) and histone deacetylase-inhibitors. | 2004 | Biochem. Pharmacol. | pmid:15183120 |
| Place RF et al. | HDAC inhibition prevents NF-kappa B activation by suppressing proteasome activity: down-regulation of proteasome subunit expression stabilizes I kappa B alpha. | 2005 | Biochem. Pharmacol. | pmid:15950952 |
| Wagner S and Roemer K | Retinoblastoma protein is required for efficient colorectal carcinoma cell apoptosis by histone deacetylase inhibitors in the absence of p21Waf. | 2005 | Biochem. Pharmacol. | pmid:15763542 |
| Vandergeeten C et al. | HIV-1 protease inhibitors do not interfere with provirus transcription and host cell apoptosis induced by combined treatment TNF-alpha + TSA. | 2007 | Biochem. Pharmacol. | pmid:17386923 |
| Carbajal A et al. | A novel method for purification of polymerizable tubulin with a high content of the acetylated isotype. | 2013 | Biochem. J. | pmid:23140207 |
| Aapola U et al. | Epigenetic modifications affect Dnmt3L expression. | 2004 | Biochem. J. | pmid:15015937 |
| Swingler TE et al. | MMP28 gene expression is regulated by Sp1 transcription factor acetylation. | 2010 | Biochem. J. | pmid:20144149 |
| Sanchez del Pino MM et al. | Properties of the yeast nuclear histone deacetylase. | 1994 | Biochem. J. | pmid:7980438 |
| Mehra-Chaudhary R et al. | Msx3 protein recruits histone deacetylase to down-regulate the Msx1 promoter. | 2001 | Biochem. J. | pmid:11115394 |
| Avram D et al. | COUP-TF (chicken ovalbumin upstream promoter transcription factor)-interacting protein 1 (CTIP1) is a sequence-specific DNA binding protein. | 2002 | Biochem. J. | pmid:12196208 |
| Arts J et al. | Stimulation of tissue-type plasminogen activator gene expression by sodium butyrate and trichostatin A in human endothelial cells involves histone acetylation. | 1995 | Biochem. J. | pmid:7646441 |
| Yang L et al. | An ERG (ets-related gene)-associated histone methyltransferase interacts with histone deacetylases 1/2 and transcription co-repressors mSin3A/B. | 2003 | Biochem. J. | pmid:12398767 |
| Hodny Z et al. | Sp1 and chromatin environment are important contributors to the formation of repressive chromatin structures on the transfected human adenine nucleotide translocase-2 promoter. | 2000 | Biochem. J. | pmid:10657244 |
| Zhang X et al. | Suppression of DPYD expression in RKO cells via DNA methylation in the regulatory region of the DPYD promoter: a potentially important epigenetic mechanism regulating DPYD expression. | 2007 | Biochem. Cell Biol. | pmid:17612628 |
| Sekhavat A et al. | Competitive inhibition of histone deacetylase activity by trichostatin A and butyrate. | 2007 | Biochem. Cell Biol. | pmid:18059533 |
| Waterborg JH and Kapros T | Kinetic analysis of histone acetylation turnover and Trichostatin A induced hyper- and hypoacetylation in alfalfa. | 2002 | Biochem. Cell Biol. | pmid:12123281 |
| Hosseinkhani M et al. | Trichostatin A induces myocardial differentiation of monkey ES cells. | 2007 | Biochem. Biophys. Res. Commun. | pmid:17368572 |
| Oger F et al. | The class I histone deacetylases of the platyhelminth parasite Schistosoma mansoni. | 2008 | Biochem. Biophys. Res. Commun. | pmid:18977200 |
| Deltour S et al. | Characterization of HRG22, a human homologue of the putative tumor suppressor gene HIC1. | 2001 | Biochem. Biophys. Res. Commun. | pmid:11554746 |
| Kishigami S et al. | Significant improvement of mouse cloning technique by treatment with trichostatin A after somatic nuclear transfer. | 2006 | Biochem. Biophys. Res. Commun. | pmid:16356478 |
| Hagiwara H et al. | Histone deacetylase inhibitor trichostatin A enhances myogenesis by coordinating muscle regulatory factors and myogenic repressors. | 2011 | Biochem. Biophys. Res. Commun. | pmid:22019851 |
| Park KS et al. | Histone modification-mediated Lhx2 gene expression. | 2012 | Biochem. Biophys. Res. Commun. | pmid:23036195 |
| Heo H et al. | Suppression of caspase-11 expression by histone deacetylase inhibitors. | 2009 | Biochem. Biophys. Res. Commun. | pmid:19013432 |
| Liu Z et al. | Deacetylase inhibitor trichostatin A down-regulates Foxp3 expression and reduces CD4+CD25+ regulatory T cells. | 2010 | Biochem. Biophys. Res. Commun. | pmid:20801101 |
| Akiba Y et al. | Histone deacetylase inhibitors de-repress tyrosine hydroxylase expression in the olfactory bulb and rostral migratory stream. | 2010 | Biochem. Biophys. Res. Commun. | pmid:20170631 |
| Bigl M et al. | Aberrant methylation of human L- and M-fructose 1,6-bisphosphatase genes in cancer. | 2008 | Biochem. Biophys. Res. Commun. | pmid:18938139 |
| Benny Klimek ME et al. | Acute inhibition of myostatin-family proteins preserves skeletal muscle in mouse models of cancer cachexia. | 2010 | Biochem. Biophys. Res. Commun. | pmid:20036643 |
| Li H and Wu X | Histone deacetylase inhibitor, Trichostatin A, activates p21WAF1/CIP1 expression through downregulation of c-myc and release of the repression of c-myc from the promoter in human cervical cancer cells. | 2004 | Biochem. Biophys. Res. Commun. | pmid:15474507 |
| Choi HS et al. | Trichostatin A, a histone deacetylase inhibitor, activates the IGFBP-3 promoter by upregulating Sp1 activity in hepatoma cells: alteration of the Sp1/Sp3/HDAC1 multiprotein complex. | 2002 | Biochem. Biophys. Res. Commun. | pmid:12200149 |
| Sowa Y et al. | Histone deacetylase inhibitor activates the WAF1/Cip1 gene promoter through the Sp1 sites. | 1997 | Biochem. Biophys. Res. Commun. | pmid:9405248 |
| Sayan BS et al. | Induction of TAp63 by histone deacetylase inhibitors. | 2010 | Biochem. Biophys. Res. Commun. | pmid:20043870 |
| Ghosh AK et al. | Trichostatin A blocks TGF-beta-induced collagen gene expression in skin fibroblasts: involvement of Sp1. | 2007 | Biochem. Biophys. Res. Commun. | pmid:17234156 |
| Matsubara K et al. | Dynamics and regulation of lysine-acetylation during one-cell stage mouse embryos. | 2013 | Biochem. Biophys. Res. Commun. | pmid:23567968 |
| Zhou X et al. | Preclinical evaluation of combined antineoplastic effect of DLC1 tumor suppressor protein and suberoylanilide hydroxamic acid on prostate cancer cells. | 2012 | Biochem. Biophys. Res. Commun. | pmid:22425986 |
| Choi S et al. | Histone deacetylases inhibitor trichostatin A modulates the extracellular release of APE1/Ref-1. | 2013 | Biochem. Biophys. Res. Commun. | pmid:23665318 |
| Maehara K et al. | Effects of histone acetylation on transcriptional regulation of manganese superoxide dismutase gene. | 2002 | Biochem. Biophys. Res. Commun. | pmid:12083788 |
| Wang XQ et al. | Histone deacetylase inhibition results in decreased macrophage CD9 expression. | 2002 | Biochem. Biophys. Res. Commun. | pmid:12056820 |
| Hu JF et al. | The role of histone acetylation in the allelic expression of the imprinted human insulin-like growth factor II gene. | 1998 | Biochem. Biophys. Res. Commun. | pmid:9792787 |
| Korhonen P et al. | Expression of transcriptional repressor protein mSin3A but not mSin3B is induced during neuronal apoptosis. | 1998 | Biochem. Biophys. Res. Commun. | pmid:9813182 |
| Hirose N et al. | ATF-2 regulates lipopolysaccharide-induced transcription in macrophage cells. | 2009 | Biochem. Biophys. Res. Commun. | pmid:19422799 |
| Kim HS et al. | Regulation of the tyrosine hydroxylase gene promoter by histone deacetylase inhibitors. | 2003 | Biochem. Biophys. Res. Commun. | pmid:14651963 |
| Sun P et al. | Effect of trichostatin A on Burkitt's lymphoma cells: Inhibition of EPS8 activity through Phospho-Erk1/2 pathway. | 2018 | Biochem. Biophys. Res. Commun. | pmid:29462617 |
| Kusakabe M et al. | Impact of DNA demethylation of the G0S2 gene on the transcription of G0S2 in squamous lung cancer cell lines with or without nuclear receptor agonists. | 2009 | Biochem. Biophys. Res. Commun. | pmid:19878646 |
| Lu ZP et al. | Histone deacetylase inhibitor Trichostatin A reduces anti-DNA autoantibody production and represses IgH gene transcription. | 2005 | Biochem. Biophys. Res. Commun. | pmid:15781251 |
| Iacobazzi V et al. | Epigenetic mechanisms and Sp1 regulate mitochondrial citrate carrier gene expression. | 2008 | Biochem. Biophys. Res. Commun. | pmid:18706393 |