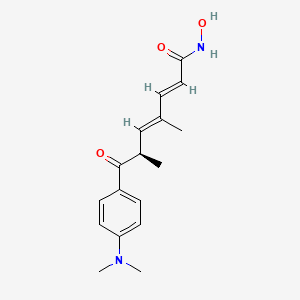
trichostatin A
Trichostatin is a lipid of Polyketides (PK) class. Trichostatin is associated with abnormalities such as Dentatorubral-Pallidoluysian Atrophy, PARAGANGLIOMAS 3, abnormal fragmented structure, Disintegration (morphologic abnormality) and Hyperostosis, Diffuse Idiopathic Skeletal. The involved functions are known as Acetylation, Cell Differentiation process, histone modification, Gene Silencing and Transcriptional Activation. Trichostatin often locates in CD41a, Hematopoietic System, Chromatin Structure, Blood and Endothelium. The associated genes with Trichostatin are SPI1 gene, CELL Gene, Chromatin, CXCR4 gene and DNMT1 gene. The related lipids are Butyrates, Promega, butyrate, Lipopolysaccharides and Steroids. The related experimental models are Knock-out, Mouse Model, Xenograft Model and Cancer Model.
Cross Reference
Introduction
To understand associated biological information of trichostatin A, we collected biological information of abnormalities, associated pathways, cellular/molecular locations, biological functions, related genes/proteins, lipids and common seen animal/experimental models with organized paragraphs from literatures.
What diseases are associated with trichostatin A?
trichostatin A is suspected in Infection, Morphologically altered structure, Ureteral obstruction, Photosensitization, Atherosclerosis, Hypertrophic Cardiomyopathy and other diseases in descending order of the highest number of associated sentences.
Related references are mostly published in these journals:
| Disease | Cross reference | Weighted score | Related literature |
|---|
Possible diseases from mapped MeSH terms on references
We collected disease MeSH terms mapped to the references associated with trichostatin A
PubChem Associated disorders and diseases
What pathways are associated with trichostatin A
Lipid pathways are not clear in current pathway databases. We organized associated pathways with trichostatin A through full-text articles, including metabolic pathways or pathways of biological mechanisms.
Related references are published most in these journals:
| Pathway name | Related literatures |
|---|
PubChem Biomolecular Interactions and Pathways
Link to PubChem Biomolecular Interactions and PathwaysWhat cellular locations are associated with trichostatin A?
Visualization in cellular structure
Associated locations are in red color. Not associated locations are in black.
Related references are published most in these journals:
| Location | Cross reference | Weighted score | Related literatures |
|---|
What functions are associated with trichostatin A?
Related references are published most in these journals:
| Function | Cross reference | Weighted score | Related literatures |
|---|
What lipids are associated with trichostatin A?
Related references are published most in these journals:
| Lipid concept | Cross reference | Weighted score | Related literatures |
|---|
What genes are associated with trichostatin A?
Related references are published most in these journals:
| Gene | Cross reference | Weighted score | Related literatures |
|---|
What common seen animal models are associated with trichostatin A?
Mouse Model
Mouse Model are used in the study 'Regulation of minichromosome maintenance gene family by microRNA-1296 and genistein in prostate cancer.' (Majid S et al., 2010), Mouse Model are used in the study 'Reversal of hypermethylation and reactivation of p16INK4a, RARbeta, and MGMT genes by genistein and other isoflavones from soy.' (Fang MZ et al., 2005) and Mouse Model are used in the study 'Histone deacetylase 3 mediates allergic skin inflammation by regulating expression of MCP1 protein.' (Kim Y et al., 2012).
Xenograft Model
Xenograft Model are used in the study 'Histone deacetylase inhibitors induce growth arrest and differentiation in uveal melanoma.' (Landreville S et al., 2012), Xenograft Model are used in the study 'Extended treatment with physiologic concentrations of dietary phytochemicals results in altered gene expression, reduced growth, and apoptosis of cancer cells.' (Moiseeva EP et al., 2007) and Xenograft Model are used in the study 'Retinoic acid and the histone deacetylase inhibitor trichostatin a inhibit the proliferation of human renal cell carcinoma in a xenograft tumor model.' (Touma SE et al., 2005).
Cancer Model
Cancer Model are used in the study 'Plasma pharmacokinetics and metabolism of the histone deacetylase inhibitor trichostatin a after intraperitoneal administration to mice.' (Sanderson L et al., 2004).
Related references are published most in these journals:
| Model | Cross reference | Weighted score | Related literatures |
|---|
NCBI Entrez Crosslinks
All references with trichostatin A
Download all related citations| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Malone CS et al. | B29 gene silencing in pituitary cells is regulated by its 3' enhancer. | 2006 | J. Mol. Biol. | pmid:16920149 |
| Lee JH et al. | Structural and functional studies of the yeast class II Hda1 histone deacetylase complex. | 2009 | J. Mol. Biol. | pmid:19573535 |
| Rincón-Arano H et al. | YY1 and GATA-1 interaction modulate the chicken 3'-side alpha-globin enhancer activity. | 2005 | J. Mol. Biol. | pmid:15913647 |
| Lopez-Atalaya JP et al. | Histone acetylation deficits in lymphoblastoid cell lines from patients with Rubinstein-Taybi syndrome. | 2012 | J. Med. Genet. | pmid:21984751 |
| Patel V et al. | Identification and characterization of small molecule inhibitors of a class I histone deacetylase from Plasmodium falciparum. | 2009 | J. Med. Chem. | pmid:19317450 |
| Olsen CA and Ghadiri MR | Discovery of potent and selective histone deacetylase inhibitors via focused combinatorial libraries of cyclic alpha3beta-tetrapeptides. | 2009 | J. Med. Chem. | pmid:19705846 |
| Trapp J et al. | Adenosine mimetics as inhibitors of NAD+-dependent histone deacetylases, from kinase to sirtuin inhibition. | 2006 | J. Med. Chem. | pmid:17149860 |
| Liu T et al. | Design and synthesis of a potent histone deacetylase inhibitor. | 2007 | J. Med. Chem. | pmid:17419603 |
| Ragno R et al. | 3-(4-Aroyl-1-methyl-1H-pyrrol-2-yl)-N-hydroxy-2-propenamides as a new class of synthetic histone deacetylase inhibitors. 3. Discovery of novel lead compounds through structure-based drug design and docking studies. | 2004 | J. Med. Chem. | pmid:14998325 |
| Kim DK et al. | Synthesis and biological evaluation of 3-(4-substituted-phenyl)-N-hydroxy-2-propenamides, a new class of histone deacetylase inhibitors. | 2003 | J. Med. Chem. | pmid:14667227 |
| Bergman JA et al. | Selective histone deacetylase 6 inhibitors bearing substituted urea linkers inhibit melanoma cell growth. | 2012 | J. Med. Chem. | pmid:23009203 |
| Remiszewski SW et al. | Inhibitors of human histone deacetylase: synthesis and enzyme and cellular activity of straight chain hydroxamates. | 2002 | J. Med. Chem. | pmid:11831887 |
| Mai A et al. | 3-(4-Aroyl-1-methyl-1H-2-pyrrolyl)-N-hydroxy-2-propenamides as a new class of synthetic histone deacetylase inhibitors. 2. Effect of pyrrole-C2 and/or -C4 substitutions on biological activity. | 2004 | J. Med. Chem. | pmid:14971890 |
| Baud MG et al. | Defining the mechanism of action and enzymatic selectivity of psammaplin A against its epigenetic targets. | 2012 | J. Med. Chem. | pmid:22280363 |
| Remiszewski SW et al. | N-hydroxy-3-phenyl-2-propenamides as novel inhibitors of human histone deacetylase with in vivo antitumor activity: discovery of (2E)-N-hydroxy-3-[4-[[(2-hydroxyethyl)[2-(1H-indol-3-yl)ethyl]amino]methyl]phenyl]-2-propenamide (NVP-LAQ824). | 2003 | J. Med. Chem. | pmid:14521422 |
| Jung M et al. | Amide analogues of trichostatin A as inhibitors of histone deacetylase and inducers of terminal cell differentiation. | 1999 | J. Med. Chem. | pmid:10579829 |
| He R et al. | Synthesis and biological evaluation of triazol-4-ylphenyl-bearing histone deacetylase inhibitors as anticancer agents. | 2010 | J. Med. Chem. | pmid:20055418 |
| Auzzas L et al. | Non-natural macrocyclic inhibitors of histone deacetylases: design, synthesis, and activity. | 2010 | J. Med. Chem. | pmid:21073160 |
| Massa S et al. | Synthesis and antimicrobial and cytotoxic activities of pyrrole-containing analogues of trichostatin A. | 1990 | J. Med. Chem. | pmid:2213836 |
| Huber K et al. | Novel 3-arylideneindolin-2-ones as inhibitors of NAD+ -dependent histone deacetylases (sirtuins). | 2010 | J. Med. Chem. | pmid:20030343 |
| Trunzer M et al. | Metabolic soft spot identification and compound optimization in early discovery phases using MetaSite and LC-MS/MS validation. | 2009 | J. Med. Chem. | pmid:19108654 |
| Massa S et al. | 3-(4-aroyl-1H-pyrrol-2-yl)-N-hydroxy-2-propenamides, a new class of synthetic histone deacetylase inhibitors. | 2001 | J. Med. Chem. | pmid:11405644 |
| Yu CW et al. | Quinazolin-4-one derivatives as selective histone deacetylase-6 inhibitors for the treatment of Alzheimer's disease. | 2013 | J. Med. Chem. | pmid:23905680 |
| Wittich S et al. | Structure-activity relationships on phenylalanine-containing inhibitors of histone deacetylase: in vitro enzyme inhibition, induction of differentiation, and inhibition of proliferation in Friend leukemic cells. | 2002 | J. Med. Chem. | pmid:12109913 |
| Lu Q et al. | Structure-based optimization of phenylbutyrate-derived histone deacetylase inhibitors. | 2005 | J. Med. Chem. | pmid:16107152 |
| Heltweg B et al. | Subtype selective substrates for histone deacetylases. | 2004 | J. Med. Chem. | pmid:15456267 |
| Paris M et al. | Histone deacetylase inhibitors: from bench to clinic. | 2008 | J. Med. Chem. | pmid:18247554 |
| Xiao J et al. | Discovery, synthesis, and biological evaluation of novel SMN protein modulators. | 2011 | J. Med. Chem. | pmid:21819082 |
| Casero RA and Woster PM | Recent advances in the development of polyamine analogues as antitumor agents. | 2009 | J. Med. Chem. | pmid:19534534 |
| Mai A et al. | Binding mode analysis of 3-(4-benzoyl-1-methyl-1H-2-pyrrolyl)-N-hydroxy-2-propenamide: a new synthetic histone deacetylase inhibitor inducing histone hyperacetylation, growth inhibition, and terminal cell differentiation. | 2002 | J. Med. Chem. | pmid:11960489 |
| Mai A et al. | Synthesis and biological properties of novel, uracil-containing histone deacetylase inhibitors. | 2006 | J. Med. Chem. | pmid:17004718 |
| Woo SH et al. | Structurally simple trichostatin A-like straight chain hydroxamates as potent histone deacetylase inhibitors. | 2002 | J. Med. Chem. | pmid:12061890 |
| Chen Y et al. | A series of potent and selective, triazolylphenyl-based histone deacetylases inhibitors with activity against pancreatic cancer cells and Plasmodium falciparum. | 2008 | J. Med. Chem. | pmid:18494463 |
| Glenn MP et al. | Antiproliferative and phenotype-transforming antitumor agents derived from cysteine. | 2004 | J. Med. Chem. | pmid:15163181 |
| Charrier C et al. | Synthesis and modeling of new benzofuranone histone deacetylase inhibitors that stimulate tumor suppressor gene expression. | 2009 | J. Med. Chem. | pmid:19385600 |
| Mai A et al. | Class II (IIa)-selective histone deacetylase inhibitors. 1. Synthesis and biological evaluation of novel (aryloxopropenyl)pyrrolyl hydroxyamides. | 2005 | J. Med. Chem. | pmid:15857140 |
| Miller TA et al. | Histone deacetylase inhibitors. | 2003 | J. Med. Chem. | pmid:14613312 |
| Day JA and Cohen SM | Investigating the selectivity of metalloenzyme inhibitors. | 2013 | J. Med. Chem. | pmid:24074025 |
| Mai A et al. | Discovery of (aryloxopropenyl)pyrrolyl hydroxyamides as selective inhibitors of class IIa histone deacetylase homologue HD1-A. | 2003 | J. Med. Chem. | pmid:14584932 |
| Kozikowski AP et al. | Use of the nitrile oxide cycloaddition (NOC) reaction for molecular probe generation: a new class of enzyme selective histone deacetylase inhibitors (HDACIs) showing picomolar activity at HDAC6. | 2008 | J. Med. Chem. | pmid:18642892 |
| Suzuki T et al. | Synthesis and histone deacetylase inhibitory activity of new benzamide derivatives. | 1999 | J. Med. Chem. | pmid:10425110 |
| Itoh Y et al. | Design, synthesis, structure--selectivity relationship, and effect on human cancer cells of a novel series of histone deacetylase 6-selective inhibitors. | 2007 | J. Med. Chem. | pmid:17929798 |
| Neelarapu R et al. | Design, synthesis, docking, and biological evaluation of novel diazide-containing isoxazole- and pyrazole-based histone deacetylase probes. | 2011 | J. Med. Chem. | pmid:21548582 |
| Suzuki T et al. | Highly potent and selective histone deacetylase 6 inhibitors designed based on a small-molecular substrate. | 2006 | J. Med. Chem. | pmid:16884291 |
| Marek L et al. | Histone deacetylase (HDAC) inhibitors with a novel connecting unit linker region reveal a selectivity profile for HDAC4 and HDAC5 with improved activity against chemoresistant cancer cells. | 2013 | J. Med. Chem. | pmid:23252603 |
| Bouchain G et al. | Development of potential antitumor agents. Synthesis and biological evaluation of a new set of sulfonamide derivatives as histone deacetylase inhibitors. | 2003 | J. Med. Chem. | pmid:12593661 |
| Nunes MJ et al. | Okadaic acid inhibits the trichostatin A-mediated increase of human CYP46A1 neuronal expression in a ERK1/2-Sp3-dependent pathway. | 2012 | J. Lipid Res. | pmid:22693257 |
| Clarke CJ et al. | ATRA transcriptionally induces nSMase2 through CBP/p300-mediated histone acetylation. | 2016 | J. Lipid Res. | pmid:27013100 |
| Reddy P | Editorial: HDAC inhibition begets more MDSCs. | 2012 | J. Leukoc. Biol. | pmid:22547132 |
| Rosborough BR et al. | Histone deacetylase inhibition facilitates GM-CSF-mediated expansion of myeloid-derived suppressor cells in vitro and in vivo. | 2012 | J. Leukoc. Biol. | pmid:22028329 |
| Rossi LE et al. | Histone deacetylase inhibitors impair NK cell viability and effector functions through inhibition of activation and receptor expression. | 2012 | J. Leukoc. Biol. | pmid:22124136 |
| Halili MA et al. | Differential effects of selective HDAC inhibitors on macrophage inflammatory responses to the Toll-like receptor 4 agonist LPS. | 2010 | J. Leukoc. Biol. | pmid:20200406 |
| Claus R et al. | Inhibitors of DNA methylation and histone deacetylation independently relieve AML1/ETO-mediated lysozyme repression. | 2006 | J. Leukoc. Biol. | pmid:17000900 |
| Bosisio D et al. | Blocking TH17-polarizing cytokines by histone deacetylase inhibitors in vitro and in vivo. | 2008 | J. Leukoc. Biol. | pmid:18780875 |
| Moldenhauer A et al. | Histone deacetylase inhibition improves dendritic cell differentiation of leukemic blasts with AML1-containing fusion proteins. | 2004 | J. Leukoc. Biol. | pmid:15197237 |
| Phelan MW et al. | Hypoxia increases thrombospondin-1 transcript and protein in cultured endothelial cells. | 1998 | J. Lab. Clin. Med. | pmid:9851743 |
| Sung TY et al. | Innovative in vitro chemo-hormonal drug therapy for refractory thyroid carcinomas. | 2012 | J. Korean Med. Sci. | pmid:22787366 |
| Jung N et al. | Pharmacological unmasking microarray approach-based discovery of novel DNA methylation markers for hepatocellular carcinoma. | 2012 | J. Korean Med. Sci. | pmid:22690089 |
| Tran HT et al. | Improved therapeutic effect against leukemia by a combination of the histone methyltransferase inhibitor chaetocin and the histone deacetylase inhibitor trichostatin A. | 2013 | J. Korean Med. Sci. | pmid:23400519 |
| Russell SB et al. | Epigenetically altered wound healing in keloid fibroblasts. | 2010 | J. Invest. Dermatol. | pmid:20555348 |
| Tigges J et al. | Aryl hydrocarbon receptor repressor (AhRR) function revisited: repression of CYP1 activity in human skin fibroblasts is not related to AhRR expression. | 2013 | J. Invest. Dermatol. | pmid:22951721 |
| Markova NG et al. | Inhibition of histone deacetylation promotes abnormal epidermal differentiation and specifically suppresses the expression of the late differentiation marker profilaggrin. | 2007 | J. Invest. Dermatol. | pmid:17195011 |
| Johnson JL et al. | The desmosomal protein desmoglein 1 aids recovery of epidermal differentiation after acute UV light exposure. | 2014 | J. Invest. Dermatol. | pmid:24594668 |
| Schauber J et al. | Histone acetylation in keratinocytes enables control of the expression of cathelicidin and CD14 by 1,25-dihydroxyvitamin D3. | 2008 | J. Invest. Dermatol. | pmid:17943182 |
| Nakazawa Y et al. | Evaluation of long-term transgene expression in piggyBac-modified human T lymphocytes. | 2013 | J. Immunother. | pmid:23211626 |
| Lu L et al. | Role of SMAD and non-SMAD signals in the development of Th17 and regulatory T cells. | 2010 | J. Immunol. | pmid:20304828 |
| Lee KY et al. | NF-kappaB and activator protein 1 response elements and the role of histone modifications in IL-1beta-induced TGF-beta1 gene transcription. | 2006 | J. Immunol. | pmid:16365456 |
| Bai Y et al. | Protein acetylation regulates both PU.1 transactivation and Ig kappa 3' enhancer activity. | 2005 | J. Immunol. | pmid:16210620 |
| Gao B et al. | Inhibition of histone deacetylase activity suppresses IFN-γ induction of tripartite motif 22 via CHIP-mediated proteasomal degradation of IRF-1. | 2013 | J. Immunol. | pmid:23729439 |
| Grabiec AM et al. | Histone deacetylase inhibitors suppress inflammatory activation of rheumatoid arthritis patient synovial macrophages and tissue. | 2010 | J. Immunol. | pmid:20100935 |
| Bachl J et al. | Increased transcription levels induce higher mutation rates in a hypermutating cell line. | 2001 | J. Immunol. | pmid:11290786 |
| Gays F et al. | The mouse tumor cell lines EL4 and RMA display mosaic expression of NK-related and certain other surface molecules and appear to have a common origin. | 2000 | J. Immunol. | pmid:10799866 |
| Maës J et al. | Chromatin remodeling at the Ig loci prior to V(D)J recombination. | 2001 | J. Immunol. | pmid:11441093 |
| Magner WJ et al. | Activation of MHC class I, II, and CD40 gene expression by histone deacetylase inhibitors. | 2000 | J. Immunol. | pmid:11120829 |
| Uhlenbrock F et al. | The NKG2D ligand ULBP2 is specifically regulated through an invariant chain-dependent endosomal pathway. | 2014 | J. Immunol. | pmid:25024379 |
| Thomas RM et al. | Regulation of mouse mammary tumor virus env transcriptional activator initiated mammary tumor virus superantigen transcripts in lymphomas of SJL/J mice: role of Ikaros, demethylation, and chromatin structural change in the transcriptional activation of mammary tumor virus superantigen. | 2003 | J. Immunol. | pmid:12496403 |
| Serrat N et al. | The response of secondary genes to lipopolysaccharides in macrophages depends on histone deacetylase and phosphorylation of C/EBPβ. | 2014 | J. Immunol. | pmid:24307736 |
| Sebastián C et al. | Deacetylase activity is required for STAT5-dependent GM-CSF functional activity in macrophages and differentiation to dendritic cells. | 2008 | J. Immunol. | pmid:18424709 |
| Ishiguro K et al. | Cutting edge: tubulin α functions as an adaptor in NFAT-importin β interaction. | 2011 | J. Immunol. | pmid:21278340 |
| Northrop JK et al. | Cutting edge: chromatin remodeling as a molecular basis for the enhanced functionality of memory CD8 T cells. | 2008 | J. Immunol. | pmid:18606637 |
| Schmeck B et al. | Histone acetylation and flagellin are essential for Legionella pneumophila-induced cytokine expression. | 2008 | J. Immunol. | pmid:18606645 |
| Zabel MD et al. | Lymphoid transcription of the murine CD21 gene is positively regulated by histone acetylation. | 1999 | J. Immunol. | pmid:10453011 |
| Zhang Y et al. | Pro-IL-16 recruits histone deacetylase 3 to the Skp2 core promoter through interaction with transcription factor GABP. | 2008 | J. Immunol. | pmid:18097041 |
| Laribee RN and Klemsz MJ | Loss of PU.1 expression following inhibition of histone deacetylases. | 2001 | J. Immunol. | pmid:11673528 |
| Mataki H et al. | Downregulation of the microRNA-1/133a cluster enhances cancer cell migration and invasion in lung-squamous cell carcinoma via regulation of Coronin1C. | 2015 | J. Hum. Genet. | pmid:25518741 |
| Xu S et al. | Abrogation of DUSP6 by hypermethylation in human pancreatic cancer. | 2005 | J. Hum. Genet. | pmid:15824892 |
| Yoshioka H et al. | A novel in vitro system for analyzing parental allele-specific histone acetylation in genomic imprinting. | 2001 | J. Hum. Genet. | pmid:11721881 |
| Huang H et al. | Expression of P53, P21 in human lung adenocarcinoma A549 cell strains under hypoxia conditions and the effect of TSA on their expression. | 2003 | J. Huazhong Univ. Sci. Technol. Med. Sci. | pmid:15015635 |
| Li C et al. | Effect and comparison of sodium butyrate and trichostatin A on the proliferation/differentiation of K562. | 2003 | J. Huazhong Univ. Sci. Technol. Med. Sci. | pmid:14526425 |
| He J et al. | Effects of trichostatin A on HDAC8 expression, proliferation and cell cycle of Molt-4 cells. | 2006 | J. Huazhong Univ. Sci. Technol. Med. Sci. | pmid:17219959 |
| Liu H et al. | Trichostatin A regulates hGCN5 expression and cell cycle on Daudi cells in vitro. | 2006 | J. Huazhong Univ. Sci. Technol. Med. Sci. | pmid:17219960 |
| Sun C et al. | Anticancer activities of trichostatin A on maligant lymphoid cells. | 2006 | J. Huazhong Univ. Sci. Technol. Med. Sci. | pmid:17219961 |
| Hong Z et al. | Microarray study of mechanism of trichostatin a inducing apoptosis of Molt-4 cells. | 2009 | J. Huazhong Univ. Sci. Technol. Med. Sci. | pmid:19662360 |
| Li X et al. | Regulation of histone acetylation and apoptosis by trichostatin in HL-60 cells. | 2004 | J. Huazhong Univ. Sci. Technol. Med. Sci. | pmid:15791844 |
| Li C et al. | Induction of myelogenous leukemia cells with histone deacetylase inhibitors through down-regulating the Daxx protein expression. | 2009 | J. Huazhong Univ. Sci. Technol. Med. Sci. | pmid:19821084 |
| Marquardt JU et al. | Curcumin effectively inhibits oncogenic NF-κB signaling and restrains stemness features in liver cancer. | 2015 | J. Hepatol. | pmid:25937435 |
| Rombouts K et al. | Actin filament formation, reorganization and migration are impaired in hepatic stellate cells under influence of trichostatin A, a histone deacetylase inhibitor. | 2002 | J. Hepatol. | pmid:12445420 |
| Papeleu P et al. | Trichostatin A induces differential cell cycle arrests but does not induce apoptosis in primary cultures of mitogen-stimulated rat hepatocytes. | 2003 | J. Hepatol. | pmid:12927923 |
| Zhang C et al. | Epigenetic inactivation of the tumor suppressor gene RIZ1 in hepatocellular carcinoma involves both DNA methylation and histone modifications. | 2010 | J. Hepatol. | pmid:20675009 |
| Chiba T et al. | Identification of genes up-regulated by histone deacetylase inhibition with cDNA microarray and exploration of epigenetic alterations on hepatoma cells. | 2004 | J. Hepatol. | pmid:15336447 |