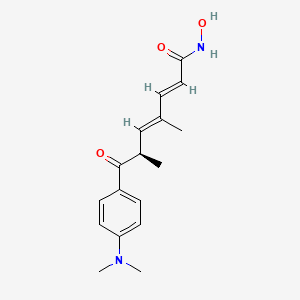
trichostatin A
Trichostatin is a lipid of Polyketides (PK) class. Trichostatin is associated with abnormalities such as Dentatorubral-Pallidoluysian Atrophy, PARAGANGLIOMAS 3, abnormal fragmented structure, Disintegration (morphologic abnormality) and Hyperostosis, Diffuse Idiopathic Skeletal. The involved functions are known as Acetylation, Cell Differentiation process, histone modification, Gene Silencing and Transcriptional Activation. Trichostatin often locates in CD41a, Hematopoietic System, Chromatin Structure, Blood and Endothelium. The associated genes with Trichostatin are SPI1 gene, CELL Gene, Chromatin, CXCR4 gene and DNMT1 gene. The related lipids are Butyrates, Promega, butyrate, Lipopolysaccharides and Steroids. The related experimental models are Knock-out, Mouse Model, Xenograft Model and Cancer Model.
Cross Reference
Introduction
To understand associated biological information of trichostatin A, we collected biological information of abnormalities, associated pathways, cellular/molecular locations, biological functions, related genes/proteins, lipids and common seen animal/experimental models with organized paragraphs from literatures.
What diseases are associated with trichostatin A?
trichostatin A is suspected in Infection, Morphologically altered structure, Ureteral obstruction, Photosensitization, Atherosclerosis, Hypertrophic Cardiomyopathy and other diseases in descending order of the highest number of associated sentences.
Related references are mostly published in these journals:
| Disease | Cross reference | Weighted score | Related literature |
|---|
Possible diseases from mapped MeSH terms on references
We collected disease MeSH terms mapped to the references associated with trichostatin A
PubChem Associated disorders and diseases
What pathways are associated with trichostatin A
Lipid pathways are not clear in current pathway databases. We organized associated pathways with trichostatin A through full-text articles, including metabolic pathways or pathways of biological mechanisms.
Related references are published most in these journals:
| Pathway name | Related literatures |
|---|
PubChem Biomolecular Interactions and Pathways
Link to PubChem Biomolecular Interactions and PathwaysWhat cellular locations are associated with trichostatin A?
Visualization in cellular structure
Associated locations are in red color. Not associated locations are in black.
Related references are published most in these journals:
| Location | Cross reference | Weighted score | Related literatures |
|---|
What functions are associated with trichostatin A?
Related references are published most in these journals:
| Function | Cross reference | Weighted score | Related literatures |
|---|
What lipids are associated with trichostatin A?
Related references are published most in these journals:
| Lipid concept | Cross reference | Weighted score | Related literatures |
|---|
What genes are associated with trichostatin A?
Related references are published most in these journals:
| Gene | Cross reference | Weighted score | Related literatures |
|---|
What common seen animal models are associated with trichostatin A?
Mouse Model
Mouse Model are used in the study 'Regulation of minichromosome maintenance gene family by microRNA-1296 and genistein in prostate cancer.' (Majid S et al., 2010), Mouse Model are used in the study 'Reversal of hypermethylation and reactivation of p16INK4a, RARbeta, and MGMT genes by genistein and other isoflavones from soy.' (Fang MZ et al., 2005) and Mouse Model are used in the study 'Histone deacetylase 3 mediates allergic skin inflammation by regulating expression of MCP1 protein.' (Kim Y et al., 2012).
Xenograft Model
Xenograft Model are used in the study 'Histone deacetylase inhibitors induce growth arrest and differentiation in uveal melanoma.' (Landreville S et al., 2012), Xenograft Model are used in the study 'Extended treatment with physiologic concentrations of dietary phytochemicals results in altered gene expression, reduced growth, and apoptosis of cancer cells.' (Moiseeva EP et al., 2007) and Xenograft Model are used in the study 'Retinoic acid and the histone deacetylase inhibitor trichostatin a inhibit the proliferation of human renal cell carcinoma in a xenograft tumor model.' (Touma SE et al., 2005).
Cancer Model
Cancer Model are used in the study 'Plasma pharmacokinetics and metabolism of the histone deacetylase inhibitor trichostatin a after intraperitoneal administration to mice.' (Sanderson L et al., 2004).
Related references are published most in these journals:
| Model | Cross reference | Weighted score | Related literatures |
|---|
NCBI Entrez Crosslinks
All references with trichostatin A
Download all related citations| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Turovets N et al. | Human parthenogenetic stem cells produce enriched populations of definitive endoderm cells after trichostatin A pretreatment. | 2011 | Differentiation | pmid:21306817 |
| Zhou X et al. | Trichostatin differentially regulates Th1 and Th2 responses and alleviates rheumatoid arthritis in mice. | 2011 | J. Clin. Immunol. | pmid:21305388 |
| Furumai R et al. | Histone deacetylase inhibitors block nuclear factor-κB-dependent transcription by interfering with RNA polymerase II recruitment. | 2011 | Cancer Sci. | pmid:21299717 |
| Bogaard HJ et al. | Suppression of histone deacetylases worsens right ventricular dysfunction after pulmonary artery banding in rats. | 2011 | Am. J. Respir. Crit. Care Med. | pmid:21297075 |
| Puppin C et al. | In thyroid cancer cell lines expression of periostin gene is controlled by p73 and is not related to epigenetic marks of active transcription. | 2011 | Cell Oncol (Dordr) | pmid:21290209 |
| MacTavish H et al. | Enhancement of vaccinia virus based oncolysis with histone deacetylase inhibitors. | 2010 | PLoS ONE | pmid:21283510 |
| Nakamura J et al. | Expression of hypoxic marker CA IX is regulated by site-specific DNA methylation and is associated with the histology of gastric cancer. | 2011 | Am. J. Pathol. | pmid:21281785 |
| Ishiguro K et al. | Cutting edge: tubulin α functions as an adaptor in NFAT-importin β interaction. | 2011 | J. Immunol. | pmid:21278340 |
| Cai HH et al. | Aberrant methylation frequency of TNFRSF10C promoter in pancreatic cancer cell lines. | 2011 | HBPD INT | pmid:21269942 |
| Jee BC et al. | Effect of trichostatin A on fertilization and embryo development during extended culture of mouse oocyte. | 2012 | Zygote | pmid:21269543 |
| Pianta A et al. | Nucleophosmin delocalization in thyroid tumour cells. | 2011 | Endocr. Pathol. | pmid:21258971 |
| Choi E et al. | Structure and property based design, synthesis and biological evaluation of γ-lactam based HDAC inhibitors. | 2011 | Bioorg. Med. Chem. Lett. | pmid:21256006 |
| Yoon CY et al. | The histone deacetylase inhibitor trichostatin A synergistically resensitizes a cisplatin resistant human bladder cancer cell line. | 2011 | J. Urol. | pmid:21255805 |
| Lee SC et al. | HDAC activity is required for efficient core promoter function at the mouse mammary tumor virus promoter. | 2011 | J. Biomed. Biotechnol. | pmid:21253530 |
| Zou CF et al. | Re-expression of ARHI (DIRAS3) induces autophagy in breast cancer cells and enhances the inhibitory effect of paclitaxel. | 2011 | BMC Cancer | pmid:21244707 |
| Hai T et al. | Pluripotency maintenance in mouse somatic cell nuclear transfer embryos and its improvement by treatment with the histone deacetylase inhibitor TSA. | 2011 | Cell Reprogram | pmid:21241188 |
| Lei L et al. | Epigenetic repression of SATB1 by polycomb group protein EZH2 in epithelial cells. | 2010 | Chin. Med. Sci. J. | pmid:21232178 |
| Mizuno S et al. | Inhibition of histone deacetylase causes emphysema. | 2011 | Am. J. Physiol. Lung Cell Mol. Physiol. | pmid:21224215 |
| Nandakumar V et al. | (-)-Epigallocatechin-3-gallate reactivates silenced tumor suppressor genes, Cip1/p21 and p16INK4a, by reducing DNA methylation and increasing histones acetylation in human skin cancer cells. | 2011 | Carcinogenesis | pmid:21209038 |
| Farinas B and Mas P | Functional implication of the MYB transcription factor RVE8/LCL5 in the circadian control of histone acetylation. | 2011 | Plant J. | pmid:21205033 |
| Erb TM et al. | Paracrine and epigenetic control of trophectoderm differentiation from human embryonic stem cells: the role of bone morphogenic protein 4 and histone deacetylases. | 2011 | Stem Cells Dev. | pmid:21204619 |
| Kitano A et al. | Therapeutic potential of trichostatin A to control inflammatory and fibrogenic disorders of the ocular surface. | 2010 | Mol. Vis. | pmid:21203344 |
| Liao M et al. | Coactivator function of positive cofactor 4 (PC4) in Sp1-directed luteinizing hormone receptor (LHR) gene transcription. | 2011 | J. Biol. Chem. | pmid:21193408 |
| Mitmaker EJ et al. | Modulation of matrix metalloproteinase activity in human thyroid cancer cell lines using demethylating agents and histone deacetylase inhibitors. | 2011 | Surgery | pmid:21193210 |
| Kang H and Roh S | Extended exposure to trichostatin A after activation alters the expression of genes important for early development in nuclear transfer murine embryos. | 2011 | J. Vet. Med. Sci. | pmid:21187677 |
| Stoppani E et al. | Point mutated caveolin-3 form (P104L) impairs myoblast differentiation via Akt and p38 signalling reduction, leading to an immature cell signature. | 2011 | Biochim. Biophys. Acta | pmid:21182936 |
| Yin H et al. | Histonedeacetylase inhibitor Oxamflatin increase HIV-1 transcription by inducing histone modification in latently infected cells. | 2011 | Mol. Biol. Rep. | pmid:21181272 |
| Spin JM et al. | Chromatin remodeling pathways in smooth muscle cell differentiation, and evidence for an integral role for p300. | 2010 | PLoS ONE | pmid:21179216 |
| Trtková K et al. | Formation of AR-SMRT binding in prostate cancer cells treated with natural histone deacetylase inhibitor. | 2010 | Cancer Biomark | pmid:21178266 |
| Tsai PF et al. | Interplay between PKCδ and Sp1 on histone deacetylase inhibitor-mediated Epstein-Barr virus reactivation. | 2011 | J. Virol. | pmid:21159880 |
| Puppin C et al. | Histone deacetylase inhibitors control the transcription and alternative splicing of prohibitin in thyroid tumor cells. | 2011 | Oncol. Rep. | pmid:21152868 |
| Shiratsuki S et al. | Trichostatin A-treated eight-cell bovine embryos had increased histone acetylation and gene expression, with increased cell numbers at the blastocyst stage. | 2011 | Theriogenology | pmid:21144563 |
| Wang YS et al. | Production of cloned calves by combination treatment of both donor cells and early cloned embryos with 5-aza-2/-deoxycytidine and trichostatin A. | 2011 | Theriogenology | pmid:21144561 |
| Gao A et al. | Epigenetic modification involved in benzene-induced apoptosis through regulating apoptosis-related genes expression. | 2011 | Cell Biol. Int. | pmid:21143203 |
| Ma AN et al. | Histone deacetylation directs DNA methylation in survivin gene silencing. | 2011 | Biochem. Biophys. Res. Commun. | pmid:21130077 |
| Vrzal R et al. | Valproic acid augments vitamin D receptor-mediated induction of CYP24 by vitamin D3: a possible cause of valproic acid-induced osteomalacia? | 2011 | Toxicol. Lett. | pmid:21115105 |
| Pabba C et al. | Design and synthesis of aryl ether and sulfone hydroxamic acids as potent histone deacetylase (HDAC) inhibitors. | 2011 | Bioorg. Med. Chem. Lett. | pmid:21109435 |
| Bayles R et al. | Histone modifications regulate the norepinephrine transporter gene. | 2010 | Cell Cycle | pmid:21088495 |
| Chakravarty G et al. | Cytoplasmic compartmentalization of SOX9 abrogates the growth arrest response of breast cancer cells that can be rescued by trichostatin A treatment. | 2011 | Cancer Biol. Ther. | pmid:21084857 |
| Muralidhar SA et al. | Histone deacetylase 9 activates gamma-globin gene expression in primary erythroid cells. | 2011 | J. Biol. Chem. | pmid:21078662 |
| Peidis P et al. | HDAC pharmacological inhibition promotes cell death through the eIF2α kinases PKR and GCN2. | 2010 | Aging (Albany NY) | pmid:21076179 |
| Wang JP et al. | Trichostatin A inhibits TGF-β1 induced in vitro chondrogenesis of hMSCs through Sp1 suppression. | 2011 | Differentiation | pmid:21074928 |
| Auzzas L et al. | Non-natural macrocyclic inhibitors of histone deacetylases: design, synthesis, and activity. | 2010 | J. Med. Chem. | pmid:21073160 |
| Nicodeme E et al. | Suppression of inflammation by a synthetic histone mimic. | 2010 | Nature | pmid:21068722 |
| Fu J et al. | Discovery of 1H-benzo[d][1,2,3]triazol-1-yl 3,4,5-trimethoxybenzoate as a potential antiproliferative agent by inhibiting histone deacetylase. | 2010 | Bioorg. Med. Chem. | pmid:21067930 |
| Marrazzo A et al. | Antiproliferative activity of phenylbutyrate ester of haloperidol metabolite II [(±)-MRJF4] in prostate cancer cells. | 2011 | Eur J Med Chem | pmid:21055848 |
| Giles KE et al. | Chromatin boundaries, insulators, and long-range interactions in the nucleus. | 2010 | Cold Spring Harb. Symp. Quant. Biol. | pmid:21047907 |
| Coleman SL et al. | Unsaturated fatty acids repress the expression of adipocyte fatty acid binding protein via the modulation of histone deacetylation in RAW 264.7 macrophages. | 2011 | Eur J Nutr | pmid:21046125 |
| Su Y et al. | Human RecQL4 helicase plays critical roles in prostate carcinogenesis. | 2010 | Cancer Res. | pmid:21045146 |
| Oyer JA et al. | Aberrantly silenced promoters retain a persistent memory of the silenced state after long-term reactivation. | 2011 | Mutat. Res. | pmid:21035468 |