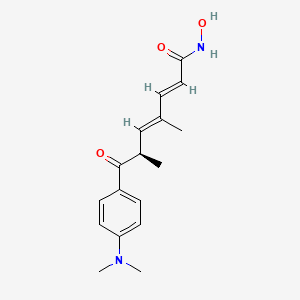
trichostatin A
Trichostatin is a lipid of Polyketides (PK) class. Trichostatin is associated with abnormalities such as Dentatorubral-Pallidoluysian Atrophy, PARAGANGLIOMAS 3, abnormal fragmented structure, Disintegration (morphologic abnormality) and Hyperostosis, Diffuse Idiopathic Skeletal. The involved functions are known as Acetylation, Cell Differentiation process, histone modification, Gene Silencing and Transcriptional Activation. Trichostatin often locates in CD41a, Hematopoietic System, Chromatin Structure, Blood and Endothelium. The associated genes with Trichostatin are SPI1 gene, CELL Gene, Chromatin, CXCR4 gene and DNMT1 gene. The related lipids are Butyrates, Promega, butyrate, Lipopolysaccharides and Steroids. The related experimental models are Knock-out, Mouse Model, Xenograft Model and Cancer Model.
Cross Reference
Introduction
To understand associated biological information of trichostatin A, we collected biological information of abnormalities, associated pathways, cellular/molecular locations, biological functions, related genes/proteins, lipids and common seen animal/experimental models with organized paragraphs from literatures.
What diseases are associated with trichostatin A?
trichostatin A is suspected in Infection, Morphologically altered structure, Ureteral obstruction, Photosensitization, Atherosclerosis, Hypertrophic Cardiomyopathy and other diseases in descending order of the highest number of associated sentences.
Related references are mostly published in these journals:
| Disease | Cross reference | Weighted score | Related literature |
|---|
Possible diseases from mapped MeSH terms on references
We collected disease MeSH terms mapped to the references associated with trichostatin A
PubChem Associated disorders and diseases
What pathways are associated with trichostatin A
Lipid pathways are not clear in current pathway databases. We organized associated pathways with trichostatin A through full-text articles, including metabolic pathways or pathways of biological mechanisms.
Related references are published most in these journals:
| Pathway name | Related literatures |
|---|
PubChem Biomolecular Interactions and Pathways
Link to PubChem Biomolecular Interactions and PathwaysWhat cellular locations are associated with trichostatin A?
Visualization in cellular structure
Associated locations are in red color. Not associated locations are in black.
Related references are published most in these journals:
| Location | Cross reference | Weighted score | Related literatures |
|---|
What functions are associated with trichostatin A?
Related references are published most in these journals:
| Function | Cross reference | Weighted score | Related literatures |
|---|
What lipids are associated with trichostatin A?
Related references are published most in these journals:
| Lipid concept | Cross reference | Weighted score | Related literatures |
|---|
What genes are associated with trichostatin A?
Related references are published most in these journals:
| Gene | Cross reference | Weighted score | Related literatures |
|---|
What common seen animal models are associated with trichostatin A?
Mouse Model
Mouse Model are used in the study 'Regulation of minichromosome maintenance gene family by microRNA-1296 and genistein in prostate cancer.' (Majid S et al., 2010), Mouse Model are used in the study 'Reversal of hypermethylation and reactivation of p16INK4a, RARbeta, and MGMT genes by genistein and other isoflavones from soy.' (Fang MZ et al., 2005) and Mouse Model are used in the study 'Histone deacetylase 3 mediates allergic skin inflammation by regulating expression of MCP1 protein.' (Kim Y et al., 2012).
Xenograft Model
Xenograft Model are used in the study 'Histone deacetylase inhibitors induce growth arrest and differentiation in uveal melanoma.' (Landreville S et al., 2012), Xenograft Model are used in the study 'Extended treatment with physiologic concentrations of dietary phytochemicals results in altered gene expression, reduced growth, and apoptosis of cancer cells.' (Moiseeva EP et al., 2007) and Xenograft Model are used in the study 'Retinoic acid and the histone deacetylase inhibitor trichostatin a inhibit the proliferation of human renal cell carcinoma in a xenograft tumor model.' (Touma SE et al., 2005).
Cancer Model
Cancer Model are used in the study 'Plasma pharmacokinetics and metabolism of the histone deacetylase inhibitor trichostatin a after intraperitoneal administration to mice.' (Sanderson L et al., 2004).
Related references are published most in these journals:
| Model | Cross reference | Weighted score | Related literatures |
|---|
NCBI Entrez Crosslinks
All references with trichostatin A
Download all related citations| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Amor DJ et al. | Human centromere repositioning "in progress". | 2004 | Proc. Natl. Acad. Sci. U.S.A. | pmid:15084747 |
| Dar RD et al. | Transcriptional burst frequency and burst size are equally modulated across the human genome. | 2012 | Proc. Natl. Acad. Sci. U.S.A. | pmid:23064634 |
| Akiyama T et al. | Inadequate histone deacetylation during oocyte meiosis causes aneuploidy and embryo death in mice. | 2006 | Proc. Natl. Acad. Sci. U.S.A. | pmid:16651529 |
| Grisolano JL et al. | An activated receptor tyrosine kinase, TEL/PDGFbetaR, cooperates with AML1/ETO to induce acute myeloid leukemia in mice. | 2003 | Proc. Natl. Acad. Sci. U.S.A. | pmid:12881486 |
| Ohno Y et al. | Macrophage inflammatory protein-2: chromosomal regulation in rat small intestinal epithelial cells. | 1997 | Proc. Natl. Acad. Sci. U.S.A. | pmid:9294201 |
| Condon JC et al. | A decline in the levels of progesterone receptor coactivators in the pregnant uterus at term may antagonize progesterone receptor function and contribute to the initiation of parturition. | 2003 | Proc. Natl. Acad. Sci. U.S.A. | pmid:12886011 |
| Slack MD et al. | Characterizing heterogeneous cellular responses to perturbations. | 2008 | Proc. Natl. Acad. Sci. U.S.A. | pmid:19052231 |
| Xu D et al. | Switch from Myc/Max to Mad1/Max binding and decrease in histone acetylation at the telomerase reverse transcriptase promoter during differentiation of HL60 cells. | 2001 | Proc. Natl. Acad. Sci. U.S.A. | pmid:11274400 |
| Boucher J et al. | Insulin and insulin-like growth factor 1 receptors are required for normal expression of imprinted genes. | 2014 | Proc. Natl. Acad. Sci. U.S.A. | pmid:25246545 |
| Chen WY et al. | Reactivation of silenced, virally transduced genes by inhibitors of histone deacetylase. | 1997 | Proc. Natl. Acad. Sci. U.S.A. | pmid:9159154 |
| Chen WY and Townes TM | Molecular mechanism for silencing virally transduced genes involves histone deacetylation and chromatin condensation. | 2000 | Proc. Natl. Acad. Sci. U.S.A. | pmid:10618426 |
| Magdinier F and Wolffe AP | Selective association of the methyl-CpG binding protein MBD2 with the silent p14/p16 locus in human neoplasia. | 2001 | Proc. Natl. Acad. Sci. U.S.A. | pmid:11309512 |
| Ma P et al. | Compensatory functions of histone deacetylase 1 (HDAC1) and HDAC2 regulate transcription and apoptosis during mouse oocyte development. | 2012 | Proc. Natl. Acad. Sci. U.S.A. | pmid:22223663 |
| Mishra N et al. | Trichostatin A reverses skewed expression of CD154, interleukin-10, and interferon-gamma gene and protein expression in lupus T cells. | 2001 | Proc. Natl. Acad. Sci. U.S.A. | pmid:11226290 |
| Zhang L et al. | Small molecule regulators of autophagy identified by an image-based high-throughput screen. | 2007 | Proc. Natl. Acad. Sci. U.S.A. | pmid:18024584 |
| Cao Y et al. | Chemical modifier screen identifies HDAC inhibitors as suppressors of PKD models. | 2009 | Proc. Natl. Acad. Sci. U.S.A. | pmid:19966229 |
| Porter NJ et al. | Unusual zinc-binding mode of HDAC6-selective hydroxamate inhibitors. | 2017 | Proc. Natl. Acad. Sci. U.S.A. | pmid:29203661 |
| Thompson EM et al. | Real time imaging of transcriptional activity in live mouse preimplantation embryos using a secreted luciferase. | 1995 | Proc. Natl. Acad. Sci. U.S.A. | pmid:7877974 |
| Xu CR et al. | Histone acetylation affects expression of cellular patterning genes in the Arabidopsis root epidermis. | 2005 | Proc. Natl. Acad. Sci. U.S.A. | pmid:16176989 |
| Jin S et al. | Ecteinascidin 743, a transcription-targeted chemotherapeutic that inhibits MDR1 activation. | 2000 | Proc. Natl. Acad. Sci. U.S.A. | pmid:10841572 |
| Claassen GF and Hann SR | A role for transcriptional repression of p21CIP1 by c-Myc in overcoming transforming growth factor beta -induced cell-cycle arrest. | 2000 | Proc. Natl. Acad. Sci. U.S.A. | pmid:10920185 |
| Emiliani S et al. | Characterization of a human RPD3 ortholog, HDAC3. | 1998 | Proc. Natl. Acad. Sci. U.S.A. | pmid:9501169 |
| Duffy S et al. | Overexpression screens identify conserved dosage chromosome instability genes in yeast and human cancer. | 2016 | Proc. Natl. Acad. Sci. U.S.A. | pmid:27551064 |
| Jenster G et al. | Steroid receptor induction of gene transcription: a two-step model. | 1997 | Proc. Natl. Acad. Sci. U.S.A. | pmid:9223281 |
| Bartsch J et al. | Moderate increase in histone acetylation activates the mouse mammary tumor virus promoter and remodels its nucleosome structure. | 1996 | Proc. Natl. Acad. Sci. U.S.A. | pmid:8855250 |
| Kenneth NS et al. | TRRAP and GCN5 are used by c-Myc to activate RNA polymerase III transcription. | 2007 | Proc. Natl. Acad. Sci. U.S.A. | pmid:17848523 |
| Iezzi S et al. | Stage-specific modulation of skeletal myogenesis by inhibitors of nuclear deacetylases. | 2002 | Proc. Natl. Acad. Sci. U.S.A. | pmid:12032356 |
| Richon VM et al. | A class of hybrid polar inducers of transformed cell differentiation inhibits histone deacetylases. | 1998 | Proc. Natl. Acad. Sci. U.S.A. | pmid:9501205 |
| Ito K et al. | A molecular mechanism of action of theophylline: Induction of histone deacetylase activity to decrease inflammatory gene expression. | 2002 | Proc. Natl. Acad. Sci. U.S.A. | pmid:12070353 |
| Condreay JP et al. | Transient and stable gene expression in mammalian cells transduced with a recombinant baculovirus vector. | 1999 | Proc. Natl. Acad. Sci. U.S.A. | pmid:9874783 |
| Crump NT et al. | Dynamic acetylation of all lysine-4 trimethylated histone H3 is evolutionarily conserved and mediated by p300/CBP. | 2011 | Proc. Natl. Acad. Sci. U.S.A. | pmid:21518915 |
| Selker EU | Trichostatin A causes selective loss of DNA methylation in Neurospora. | 1998 | Proc. Natl. Acad. Sci. U.S.A. | pmid:9689097 |
| Plouffe D et al. | In silico activity profiling reveals the mechanism of action of antimalarials discovered in a high-throughput screen. | 2008 | Proc. Natl. Acad. Sci. U.S.A. | pmid:18579783 |
| Saito A et al. | A synthetic inhibitor of histone deacetylase, MS-27-275, with marked in vivo antitumor activity against human tumors. | 1999 | Proc. Natl. Acad. Sci. U.S.A. | pmid:10200307 |
| Tavera-Mendoza LE et al. | Incorporation of histone deacetylase inhibition into the structure of a nuclear receptor agonist. | 2008 | Proc. Natl. Acad. Sci. U.S.A. | pmid:18550844 |
| Ji Y et al. | A multistep mechanism for the activation of rearrangement in the immune system. | 2003 | Proc. Natl. Acad. Sci. U.S.A. | pmid:12802019 |
| Liu Z et al. | Steroid receptor coactivator-1 (SRC-1) enhances ligand-dependent and receptor-dependent cell-free transcription of chromatin. | 1999 | Proc. Natl. Acad. Sci. U.S.A. | pmid:10449719 |
| Jansen MS et al. | Short-chain fatty acids enhance nuclear receptor activity through mitogen-activated protein kinase activation and histone deacetylase inhibition. | 2004 | Proc. Natl. Acad. Sci. U.S.A. | pmid:15103026 |
| Kim MY et al. | A repressor complex, AP4 transcription factor and geminin, negatively regulates expression of target genes in nonneuronal cells. | 2006 | Proc. Natl. Acad. Sci. U.S.A. | pmid:16924111 |
| Monte M et al. | MAGE-A tumor antigens target p53 transactivation function through histone deacetylase recruitment and confer resistance to chemotherapeutic agents. | 2006 | Proc. Natl. Acad. Sci. U.S.A. | pmid:16847267 |
| Furumai R et al. | Potent histone deacetylase inhibitors built from trichostatin A and cyclic tetrapeptide antibiotics including trapoxin. | 2001 | Proc. Natl. Acad. Sci. U.S.A. | pmid:11134513 |
| Subramanian C et al. | Ku70 acetylation mediates neuroblastoma cell death induced by histone deacetylase inhibitors. | 2005 | Proc. Natl. Acad. Sci. U.S.A. | pmid:15778293 |
| Liberatore RA et al. | NF-kappaB activity is constitutively elevated in c-Abl null fibroblasts. | 2009 | Proc. Natl. Acad. Sci. U.S.A. | pmid:19805123 |
| Sasaki K et al. | Real-time imaging of histone H4 hyperacetylation in living cells. | 2009 | Proc. Natl. Acad. Sci. U.S.A. | pmid:19805290 |
| Gibbs A et al. | Sulforaphane destabilizes the androgen receptor in prostate cancer cells by inactivating histone deacetylase 6. | 2009 | Proc. Natl. Acad. Sci. U.S.A. | pmid:19805354 |
| McCullough SD et al. | Reelin is a target of polyglutamine expanded ataxin-7 in human spinocerebellar ataxia type 7 (SCA7) astrocytes. | 2012 | Proc. Natl. Acad. Sci. U.S.A. | pmid:23236151 |
| Weaver IC et al. | Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. | 2006 | Proc. Natl. Acad. Sci. U.S.A. | pmid:16484373 |
| Li B et al. | FOXP3 interactions with histone acetyltransferase and class II histone deacetylases are required for repression. | 2007 | Proc. Natl. Acad. Sci. U.S.A. | pmid:17360565 |
| Zhou G et al. | HSV carrying WT REST establishes latency but reactivates only if the synthesis of REST is suppressed. | 2013 | Proc. Natl. Acad. Sci. U.S.A. | pmid:23341636 |
| Terhune SS et al. | Human cytomegalovirus UL29/28 protein interacts with components of the NuRD complex which promote accumulation of immediate-early RNA. | 2010 | PLoS Pathog. | pmid:20585571 |