| MeSH term | MeSH ID | Detail |
|---|---|---|
| Roseolovirus Infections | D019349 | 1 associated lipids |
| Myopathy, Central Core | D020512 | 1 associated lipids |
| Thyroid Hormone Resistance Syndrome | D018382 | 1 associated lipids |
| Chromosome Inversion | D007446 | 1 associated lipids |
| Rhabdomyosarcoma, Embryonal | D018233 | 1 associated lipids |
| Carcinoma, Papillary, Follicular | D018265 | 1 associated lipids |
| Gestational Trophoblastic Disease | D031901 | 1 associated lipids |
| Rhabdoid Tumor | D018335 | 1 associated lipids |
| Visceral Pain | D059265 | 1 associated lipids |
| Lupus Vulgaris | D008177 | 1 associated lipids |
| Rubinstein-Taybi Syndrome | D012415 | 1 associated lipids |
| Classical Lissencephalies and Subcortical Band Heterotopias | D054221 | 1 associated lipids |
| Goldenhar Syndrome | D006053 | 1 associated lipids |
| Adenomyosis | D062788 | 1 associated lipids |
| Capsule Opacification | D058442 | 1 associated lipids |
| Neoplasm Micrometastasis | D061206 | 1 associated lipids |
| Cystadenoma, Serous | D018293 | 1 associated lipids |
| von Hippel-Lindau Disease | D006623 | 1 associated lipids |
| Intervertebral Disc Degeneration | D055959 | 1 associated lipids |
| Supratentorial Neoplasms | D015173 | 1 associated lipids |
| Hypesthesia | D006987 | 1 associated lipids |
| Cystadenocarcinoma, Mucinous | D018282 | 1 associated lipids |
| Cystadenocarcinoma, Serous | D018284 | 2 associated lipids |
| Fibromatosis, Aggressive | D018222 | 2 associated lipids |
| Small Cell Lung Carcinoma | D055752 | 2 associated lipids |
| Rhabdomyosarcoma, Alveolar | D018232 | 2 associated lipids |
| Bone Marrow Neoplasms | D019046 | 2 associated lipids |
| Adenocarcinoma, Papillary | D000231 | 2 associated lipids |
| Inflammatory Breast Neoplasms | D058922 | 2 associated lipids |
| Ganglioneuroma | D005729 | 2 associated lipids |
| Mastocytoma | D034801 | 3 associated lipids |
| Uveal Neoplasms | D014604 | 3 associated lipids |
| Leukemia, Promyelocytic, Acute | D015473 | 3 associated lipids |
| Conjunctival Neoplasms | D003230 | 3 associated lipids |
| Adenocarcinoma, Follicular | D018263 | 3 associated lipids |
| Hypereosinophilic Syndrome | D017681 | 3 associated lipids |
| Lymphoma, Follicular | D008224 | 3 associated lipids |
| Lymphoma, Large-Cell, Anaplastic | D017728 | 3 associated lipids |
| Retinal Neoplasms | D019572 | 3 associated lipids |
| Progeria | D011371 | 3 associated lipids |
| Spinocerebellar Ataxias | D020754 | 4 associated lipids |
| Nasopharyngeal Neoplasms | D009303 | 4 associated lipids |
| Neuroendocrine Tumors | D018358 | 4 associated lipids |
| Porcine Reproductive and Respiratory Syndrome | D019318 | 4 associated lipids |
| Cicatrix, Hypertrophic | D017439 | 4 associated lipids |
| Opioid-Related Disorders | D009293 | 5 associated lipids |
| Osteomalacia | D010018 | 5 associated lipids |
| Fragile X Syndrome | D005600 | 5 associated lipids |
| Myeloproliferative Disorders | D009196 | 5 associated lipids |
| Primary Myelofibrosis | D055728 | 6 associated lipids |
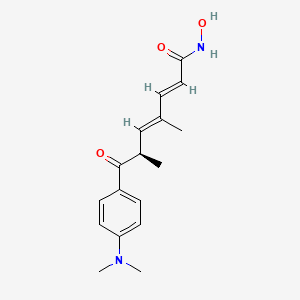
trichostatin A
Trichostatin is a lipid of Polyketides (PK) class. Trichostatin is associated with abnormalities such as Dentatorubral-Pallidoluysian Atrophy, PARAGANGLIOMAS 3, abnormal fragmented structure, Disintegration (morphologic abnormality) and Hyperostosis, Diffuse Idiopathic Skeletal. The involved functions are known as Acetylation, Cell Differentiation process, histone modification, Gene Silencing and Transcriptional Activation. Trichostatin often locates in CD41a, Hematopoietic System, Chromatin Structure, Blood and Endothelium. The associated genes with Trichostatin are SPI1 gene, CELL Gene, Chromatin, CXCR4 gene and DNMT1 gene. The related lipids are Butyrates, Promega, butyrate, Lipopolysaccharides and Steroids. The related experimental models are Knock-out, Mouse Model, Xenograft Model and Cancer Model.
Cross Reference
Introduction
To understand associated biological information of trichostatin A, we collected biological information of abnormalities, associated pathways, cellular/molecular locations, biological functions, related genes/proteins, lipids and common seen animal/experimental models with organized paragraphs from literatures.
What diseases are associated with trichostatin A?
trichostatin A is suspected in Infection, Morphologically altered structure, Ureteral obstruction, Photosensitization, Atherosclerosis, Hypertrophic Cardiomyopathy and other diseases in descending order of the highest number of associated sentences.
Related references are mostly published in these journals:
| Disease | Cross reference | Weighted score | Related literature |
|---|
Possible diseases from mapped MeSH terms on references
We collected disease MeSH terms mapped to the references associated with trichostatin A
PubChem Associated disorders and diseases
What pathways are associated with trichostatin A
Lipid pathways are not clear in current pathway databases. We organized associated pathways with trichostatin A through full-text articles, including metabolic pathways or pathways of biological mechanisms.
Related references are published most in these journals:
| Pathway name | Related literatures |
|---|
PubChem Biomolecular Interactions and Pathways
Link to PubChem Biomolecular Interactions and PathwaysWhat cellular locations are associated with trichostatin A?
Visualization in cellular structure
Associated locations are in red color. Not associated locations are in black.
Related references are published most in these journals:
| Location | Cross reference | Weighted score | Related literatures |
|---|
What functions are associated with trichostatin A?
Related references are published most in these journals:
| Function | Cross reference | Weighted score | Related literatures |
|---|
What lipids are associated with trichostatin A?
Related references are published most in these journals:
| Lipid concept | Cross reference | Weighted score | Related literatures |
|---|
What genes are associated with trichostatin A?
Related references are published most in these journals:
| Gene | Cross reference | Weighted score | Related literatures |
|---|
What common seen animal models are associated with trichostatin A?
Mouse Model
Mouse Model are used in the study 'Regulation of minichromosome maintenance gene family by microRNA-1296 and genistein in prostate cancer.' (Majid S et al., 2010), Mouse Model are used in the study 'Reversal of hypermethylation and reactivation of p16INK4a, RARbeta, and MGMT genes by genistein and other isoflavones from soy.' (Fang MZ et al., 2005) and Mouse Model are used in the study 'Histone deacetylase 3 mediates allergic skin inflammation by regulating expression of MCP1 protein.' (Kim Y et al., 2012).
Xenograft Model
Xenograft Model are used in the study 'Histone deacetylase inhibitors induce growth arrest and differentiation in uveal melanoma.' (Landreville S et al., 2012), Xenograft Model are used in the study 'Extended treatment with physiologic concentrations of dietary phytochemicals results in altered gene expression, reduced growth, and apoptosis of cancer cells.' (Moiseeva EP et al., 2007) and Xenograft Model are used in the study 'Retinoic acid and the histone deacetylase inhibitor trichostatin a inhibit the proliferation of human renal cell carcinoma in a xenograft tumor model.' (Touma SE et al., 2005).
Cancer Model
Cancer Model are used in the study 'Plasma pharmacokinetics and metabolism of the histone deacetylase inhibitor trichostatin a after intraperitoneal administration to mice.' (Sanderson L et al., 2004).
Related references are published most in these journals:
| Model | Cross reference | Weighted score | Related literatures |
|---|
NCBI Entrez Crosslinks
All references with trichostatin A
Download all related citations| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Chiocca S et al. | Histone deacetylase 1 inactivation by an adenovirus early gene product. | 2002 | Curr. Biol. | pmid:11937030 |
| Milutinovic S et al. | Proliferating cell nuclear antigen associates with histone deacetylase activity, integrating DNA replication and chromatin modification. | 2002 | J. Biol. Chem. | pmid:11929879 |
| Kamitani H et al. | Histone acetylation may suppress human glioma cell proliferation when p21 WAF/Cip1 and gelsolin are induced. | 2002 | Neuro-oncology | pmid:11916500 |
| Sawa H et al. | Histone deacetylase inhibitors such as sodium butyrate and trichostatin A induce apoptosis through an increase of the bcl-2-related protein Bad. | 2001 | Brain Tumor Pathol | pmid:11908866 |
| Heltweg B and Jung M | A microplate reader-based nonisotopic histone deacetylase activity assay. | 2002 | Anal. Biochem. | pmid:11878795 |
| Taniura S et al. | Transcriptional regulation of cyclooxygenase-1 by histone deacetylase inhibitors in normal human astrocyte cells. | 2002 | J. Biol. Chem. | pmid:11877441 |
| Murphy JC et al. | Control of cytomegalovirus lytic gene expression by histone acetylation. | 2002 | EMBO J. | pmid:11867539 |
| Drewell RA et al. | Methylation-dependent silencing at the H19 imprinting control region by MeCP2. | 2002 | Nucleic Acids Res. | pmid:11861904 |
| Hou M et al. | The histone deacetylase inhibitor trichostatin A derepresses the telomerase reverse transcriptase (hTERT) gene in human cells. | 2002 | Exp. Cell Res. | pmid:11855854 |
| McBurney MW et al. | Evidence for repeat-induced gene silencing in cultured Mammalian cells: inactivation of tandem repeats of transfected genes. | 2002 | Exp. Cell Res. | pmid:11855851 |
| Valapour M et al. | Histone deacetylation inhibits IL4 gene expression in T cells. | 2002 | J. Allergy Clin. Immunol. | pmid:11842291 |
| Roddie PH et al. | Primary acute myeloid leukaemia blasts resistant to cytokine-induced differentiation to dendritic-like leukaemia cells can be forced to differentiate by the addition of bryostatin-1. | 2002 | Leukemia | pmid:11840267 |
| Kiefer SM et al. | Murine Sall1 represses transcription by recruiting a histone deacetylase complex. | 2002 | J. Biol. Chem. | pmid:11836251 |
| Remiszewski SW et al. | Inhibitors of human histone deacetylase: synthesis and enzyme and cellular activity of straight chain hydroxamates. | 2002 | J. Med. Chem. | pmid:11831887 |
| Zhou DC et al. | Frequent mutations in the ligand-binding domain of PML-RARalpha after multiple relapses of acute promyelocytic leukemia: analysis for functional relationship to response to all-trans retinoic acid and histone deacetylase inhibitors in vitro and in vivo. | 2002 | Blood | pmid:11830487 |
| Herold C et al. | The histone-deacetylase inhibitor Trichostatin A blocks proliferation and triggers apoptotic programs in hepatoma cells. | 2002 | J. Hepatol. | pmid:11830335 |
| Handumrongkul C et al. | Distinct sets of cellular genes control the expression of transfected, nuclear-localized genes. | 2002 | Mol. Ther. | pmid:11829526 |
| Sourlingas TG et al. | Histone deacetylase inhibitors induce apoptosis in peripheral blood lymphocytes along with histone H4 acetylation and the expression of the linker histone variant, H1 degrees. | 2001 | Eur. J. Cell Biol. | pmid:11824792 |
| Deroanne CF et al. | Histone deacetylases inhibitors as anti-angiogenic agents altering vascular endothelial growth factor signaling. | 2002 | Oncogene | pmid:11821955 |
| Wilson MA et al. | The histone deacetylase inhibitor trichostatin A blocks progesterone receptor-mediated transactivation of the mouse mammary tumor virus promoter in vivo. | 2002 | J. Biol. Chem. | pmid:11821430 |