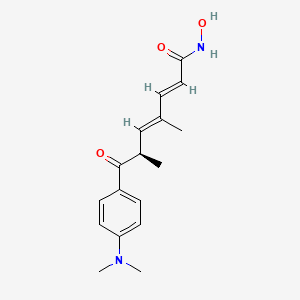
trichostatin A
Trichostatin is a lipid of Polyketides (PK) class. Trichostatin is associated with abnormalities such as Dentatorubral-Pallidoluysian Atrophy, PARAGANGLIOMAS 3, abnormal fragmented structure, Disintegration (morphologic abnormality) and Hyperostosis, Diffuse Idiopathic Skeletal. The involved functions are known as Acetylation, Cell Differentiation process, histone modification, Gene Silencing and Transcriptional Activation. Trichostatin often locates in CD41a, Hematopoietic System, Chromatin Structure, Blood and Endothelium. The associated genes with Trichostatin are SPI1 gene, CELL Gene, Chromatin, CXCR4 gene and DNMT1 gene. The related lipids are Butyrates, Promega, butyrate, Lipopolysaccharides and Steroids. The related experimental models are Knock-out, Mouse Model, Xenograft Model and Cancer Model.
Cross Reference
Introduction
To understand associated biological information of trichostatin A, we collected biological information of abnormalities, associated pathways, cellular/molecular locations, biological functions, related genes/proteins, lipids and common seen animal/experimental models with organized paragraphs from literatures.
What diseases are associated with trichostatin A?
trichostatin A is suspected in Infection, Morphologically altered structure, Ureteral obstruction, Photosensitization, Atherosclerosis, Hypertrophic Cardiomyopathy and other diseases in descending order of the highest number of associated sentences.
Related references are mostly published in these journals:
| Disease | Cross reference | Weighted score | Related literature |
|---|
Possible diseases from mapped MeSH terms on references
We collected disease MeSH terms mapped to the references associated with trichostatin A
PubChem Associated disorders and diseases
What pathways are associated with trichostatin A
Lipid pathways are not clear in current pathway databases. We organized associated pathways with trichostatin A through full-text articles, including metabolic pathways or pathways of biological mechanisms.
Related references are published most in these journals:
| Pathway name | Related literatures |
|---|
PubChem Biomolecular Interactions and Pathways
Link to PubChem Biomolecular Interactions and PathwaysWhat cellular locations are associated with trichostatin A?
Visualization in cellular structure
Associated locations are in red color. Not associated locations are in black.
Related references are published most in these journals:
| Location | Cross reference | Weighted score | Related literatures |
|---|
What functions are associated with trichostatin A?
Related references are published most in these journals:
| Function | Cross reference | Weighted score | Related literatures |
|---|
What lipids are associated with trichostatin A?
Related references are published most in these journals:
| Lipid concept | Cross reference | Weighted score | Related literatures |
|---|
What genes are associated with trichostatin A?
Related references are published most in these journals:
| Gene | Cross reference | Weighted score | Related literatures |
|---|
What common seen animal models are associated with trichostatin A?
Mouse Model
Mouse Model are used in the study 'Regulation of minichromosome maintenance gene family by microRNA-1296 and genistein in prostate cancer.' (Majid S et al., 2010), Mouse Model are used in the study 'Reversal of hypermethylation and reactivation of p16INK4a, RARbeta, and MGMT genes by genistein and other isoflavones from soy.' (Fang MZ et al., 2005) and Mouse Model are used in the study 'Histone deacetylase 3 mediates allergic skin inflammation by regulating expression of MCP1 protein.' (Kim Y et al., 2012).
Xenograft Model
Xenograft Model are used in the study 'Histone deacetylase inhibitors induce growth arrest and differentiation in uveal melanoma.' (Landreville S et al., 2012), Xenograft Model are used in the study 'Extended treatment with physiologic concentrations of dietary phytochemicals results in altered gene expression, reduced growth, and apoptosis of cancer cells.' (Moiseeva EP et al., 2007) and Xenograft Model are used in the study 'Retinoic acid and the histone deacetylase inhibitor trichostatin a inhibit the proliferation of human renal cell carcinoma in a xenograft tumor model.' (Touma SE et al., 2005).
Cancer Model
Cancer Model are used in the study 'Plasma pharmacokinetics and metabolism of the histone deacetylase inhibitor trichostatin a after intraperitoneal administration to mice.' (Sanderson L et al., 2004).
Related references are published most in these journals:
| Model | Cross reference | Weighted score | Related literatures |
|---|
NCBI Entrez Crosslinks
All references with trichostatin A
Download all related citations| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Yoshida M et al. | Histone deacetylase as a new target for cancer chemotherapy. | 2001 | Cancer Chemother. Pharmacol. | pmid:11587361 |
| Jung M | Inhibitors of histone deacetylase as new anticancer agents. | 2001 | Curr. Med. Chem. | pmid:11562279 |
| Ashburner BP et al. | The p65 (RelA) subunit of NF-kappaB interacts with the histone deacetylase (HDAC) corepressors HDAC1 and HDAC2 to negatively regulate gene expression. | 2001 | Mol. Cell. Biol. | pmid:11564889 |
| Sachs LM et al. | Involvement of histone deacetylase at two distinct steps in gene regulation during intestinal development in Xenopus laevis. | 2001 | Dev. Dyn. | pmid:11668605 |
| Amann JM et al. | ETO, a target of t(8;21) in acute leukemia, makes distinct contacts with multiple histone deacetylases and binds mSin3A through its oligomerization domain. | 2001 | Mol. Cell. Biol. | pmid:11533236 |
| Phiel CJ et al. | Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. | 2001 | J. Biol. Chem. | pmid:11473107 |
| Sundqvist A et al. | Functional knockout of the corepressor CtBP by the second exon of adenovirus E1a relieves repression of transcription. | 2001 | Exp. Cell Res. | pmid:11478854 |
| Jacob AL et al. | Acetylation of steroidogenic factor 1 protein regulates its transcriptional activity and recruits the coactivator GCN5. | 2001 | J. Biol. Chem. | pmid:11479297 |
| Li J et al. | Transcriptional induction of MKP-1 in response to stress is associated with histone H3 phosphorylation-acetylation. | 2001 | Mol. Cell. Biol. | pmid:11689710 |
| Hirschler-Laszkiewicz I et al. | The role of acetylation in rDNA transcription. | 2001 | Nucleic Acids Res. | pmid:11600700 |
| Klar AJ et al. | A histone deacetylation inhibitor and mutant promote colony-type switching of the human pathogen Candida albicans. | 2001 | Genetics | pmid:11404352 |
| Massa S et al. | 3-(4-aroyl-1H-pyrrol-2-yl)-N-hydroxy-2-propenamides, a new class of synthetic histone deacetylase inhibitors. | 2001 | J. Med. Chem. | pmid:11405644 |
| Setlow JK et al. | Evidence for gene silencing in Haemophilus influenzae. | 2001 | Mutat. Res. | pmid:11406170 |
| Singal R et al. | Cytosine methylation represses glutathione S-transferase P1 (GSTP1) gene expression in human prostate cancer cells. | 2001 | Cancer Res. | pmid:11406558 |
| Feng YQ et al. | Position effects are influenced by the orientation of a transgene with respect to flanking chromatin. | 2001 | Mol. Cell. Biol. | pmid:11113204 |
| Mehra-Chaudhary R et al. | Msx3 protein recruits histone deacetylase to down-regulate the Msx1 promoter. | 2001 | Biochem. J. | pmid:11115394 |
| Kuwahara K et al. | The neuron-restrictive silencer element-neuron-restrictive silencer factor system regulates basal and endothelin 1-inducible atrial natriuretic peptide gene expression in ventricular myocytes. | 2001 | Mol. Cell. Biol. | pmid:11238943 |
| Meier JL | Reactivation of the human cytomegalovirus major immediate-early regulatory region and viral replication in embryonal NTera2 cells: role of trichostatin A, retinoic acid, and deletion of the 21-base-pair repeats and modulator. | 2001 | J. Virol. | pmid:11160656 |
| Kuusisto E et al. | Ubiquitin-binding protein p62 expression is induced during apoptosis and proteasomal inhibition in neuronal cells. | 2001 | Biochem. Biophys. Res. Commun. | pmid:11162503 |
| Grandjean V et al. | Relationship between DNA methylation, histone H4 acetylation and gene expression in the mouse imprinted Igf2-H19 domain. | 2001 | FEBS Lett. | pmid:11163765 |