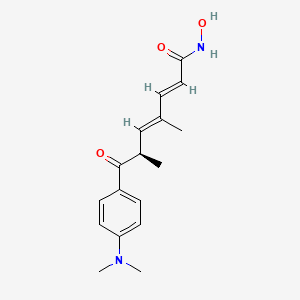
trichostatin A
Trichostatin is a lipid of Polyketides (PK) class. Trichostatin is associated with abnormalities such as Dentatorubral-Pallidoluysian Atrophy, PARAGANGLIOMAS 3, abnormal fragmented structure, Disintegration (morphologic abnormality) and Hyperostosis, Diffuse Idiopathic Skeletal. The involved functions are known as Acetylation, Cell Differentiation process, histone modification, Gene Silencing and Transcriptional Activation. Trichostatin often locates in CD41a, Hematopoietic System, Chromatin Structure, Blood and Endothelium. The associated genes with Trichostatin are SPI1 gene, CELL Gene, Chromatin, CXCR4 gene and DNMT1 gene. The related lipids are Butyrates, Promega, butyrate, Lipopolysaccharides and Steroids. The related experimental models are Knock-out, Mouse Model, Xenograft Model and Cancer Model.
Cross Reference
Introduction
To understand associated biological information of trichostatin A, we collected biological information of abnormalities, associated pathways, cellular/molecular locations, biological functions, related genes/proteins, lipids and common seen animal/experimental models with organized paragraphs from literatures.
What diseases are associated with trichostatin A?
trichostatin A is suspected in Infection, Morphologically altered structure, Ureteral obstruction, Photosensitization, Atherosclerosis, Hypertrophic Cardiomyopathy and other diseases in descending order of the highest number of associated sentences.
Related references are mostly published in these journals:
| Disease | Cross reference | Weighted score | Related literature |
|---|
Possible diseases from mapped MeSH terms on references
We collected disease MeSH terms mapped to the references associated with trichostatin A
PubChem Associated disorders and diseases
What pathways are associated with trichostatin A
Lipid pathways are not clear in current pathway databases. We organized associated pathways with trichostatin A through full-text articles, including metabolic pathways or pathways of biological mechanisms.
Related references are published most in these journals:
| Pathway name | Related literatures |
|---|
PubChem Biomolecular Interactions and Pathways
Link to PubChem Biomolecular Interactions and PathwaysWhat cellular locations are associated with trichostatin A?
Visualization in cellular structure
Associated locations are in red color. Not associated locations are in black.
Related references are published most in these journals:
| Location | Cross reference | Weighted score | Related literatures |
|---|
What functions are associated with trichostatin A?
Related references are published most in these journals:
| Function | Cross reference | Weighted score | Related literatures |
|---|
What lipids are associated with trichostatin A?
Related references are published most in these journals:
| Lipid concept | Cross reference | Weighted score | Related literatures |
|---|
What genes are associated with trichostatin A?
Related references are published most in these journals:
| Gene | Cross reference | Weighted score | Related literatures |
|---|
What common seen animal models are associated with trichostatin A?
Mouse Model
Mouse Model are used in the study 'Regulation of minichromosome maintenance gene family by microRNA-1296 and genistein in prostate cancer.' (Majid S et al., 2010), Mouse Model are used in the study 'Reversal of hypermethylation and reactivation of p16INK4a, RARbeta, and MGMT genes by genistein and other isoflavones from soy.' (Fang MZ et al., 2005) and Mouse Model are used in the study 'Histone deacetylase 3 mediates allergic skin inflammation by regulating expression of MCP1 protein.' (Kim Y et al., 2012).
Xenograft Model
Xenograft Model are used in the study 'Histone deacetylase inhibitors induce growth arrest and differentiation in uveal melanoma.' (Landreville S et al., 2012), Xenograft Model are used in the study 'Extended treatment with physiologic concentrations of dietary phytochemicals results in altered gene expression, reduced growth, and apoptosis of cancer cells.' (Moiseeva EP et al., 2007) and Xenograft Model are used in the study 'Retinoic acid and the histone deacetylase inhibitor trichostatin a inhibit the proliferation of human renal cell carcinoma in a xenograft tumor model.' (Touma SE et al., 2005).
Cancer Model
Cancer Model are used in the study 'Plasma pharmacokinetics and metabolism of the histone deacetylase inhibitor trichostatin a after intraperitoneal administration to mice.' (Sanderson L et al., 2004).
Related references are published most in these journals:
| Model | Cross reference | Weighted score | Related literatures |
|---|
NCBI Entrez Crosslinks
All references with trichostatin A
Download all related citations| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Ashburner BP et al. | The p65 (RelA) subunit of NF-kappaB interacts with the histone deacetylase (HDAC) corepressors HDAC1 and HDAC2 to negatively regulate gene expression. | 2001 | Mol. Cell. Biol. | pmid:11564889 |
| Sachs LM et al. | Involvement of histone deacetylase at two distinct steps in gene regulation during intestinal development in Xenopus laevis. | 2001 | Dev. Dyn. | pmid:11668605 |
| Amann JM et al. | ETO, a target of t(8;21) in acute leukemia, makes distinct contacts with multiple histone deacetylases and binds mSin3A through its oligomerization domain. | 2001 | Mol. Cell. Biol. | pmid:11533236 |
| Jacob AL et al. | Acetylation of steroidogenic factor 1 protein regulates its transcriptional activity and recruits the coactivator GCN5. | 2001 | J. Biol. Chem. | pmid:11479297 |
| Sourlingas TG et al. | Histone deacetylase inhibitors induce apoptosis in peripheral blood lymphocytes along with histone H4 acetylation and the expression of the linker histone variant, H1 degrees. | 2001 | Eur. J. Cell Biol. | pmid:11824792 |
| Li J et al. | Transcriptional induction of MKP-1 in response to stress is associated with histone H3 phosphorylation-acetylation. | 2001 | Mol. Cell. Biol. | pmid:11689710 |
| Hirschler-Laszkiewicz I et al. | The role of acetylation in rDNA transcription. | 2001 | Nucleic Acids Res. | pmid:11600700 |
| Nair AR et al. | Paradoxical effects of trichostatin A: inhibition of NF-Y-associated histone acetyltransferase activity, phosphorylation of hGCN5 and downregulation of cyclin A and B1 mRNA. | 2001 | Cancer Lett. | pmid:11295287 |
| Hatama S et al. | Reactivation of feline foamy virus from a chronically infected feline renal cell line by trichostatin A. | 2001 | Virology | pmid:11336556 |
| Viollet B et al. | Embryonic but not postnatal reexpression of hepatocyte nuclear factor 1alpha (HNF1alpha) can reactivate the silent phenylalanine hydroxylase gene in HNF1alpha-deficient hepatocytes. | 2001 | Mol. Cell. Biol. | pmid:11340160 |
| Nakamura M et al. | Reduction of telomerase activity in human liver cancer cells by a histone deacetylase inhibitor. | 2001 | J. Cell. Physiol. | pmid:11319763 |
| Xu D et al. | Switch from Myc/Max to Mad1/Max binding and decrease in histone acetylation at the telomerase reverse transcriptase promoter during differentiation of HL60 cells. | 2001 | Proc. Natl. Acad. Sci. U.S.A. | pmid:11274400 |
| Seth KA and Majzoub JA | Repressor element silencing transcription factor/neuron-restrictive silencing factor (REST/NRSF) can act as an enhancer as well as a repressor of corticotropin-releasing hormone gene transcription. | 2001 | J. Biol. Chem. | pmid:11278361 |
| Setlow JK et al. | Evidence for gene silencing in Haemophilus influenzae. | 2001 | Mutat. Res. | pmid:11406170 |
| Singal R et al. | Cytosine methylation represses glutathione S-transferase P1 (GSTP1) gene expression in human prostate cancer cells. | 2001 | Cancer Res. | pmid:11406558 |
| Baur JA et al. | Telomere position effect in human cells. | 2001 | Science | pmid:11408657 |
| Zelivianski S et al. | Multipathways for transdifferentiation of human prostate cancer cells into neuroendocrine-like phenotype. | 2001 | Biochim. Biophys. Acta | pmid:11389966 |
| Harada Y et al. | A hematopoietic-specific transmembrane protein, Art-1, is possibly regulated by AML1. | 2001 | Biochem. Biophys. Res. Commun. | pmid:11396961 |
| Yang H et al. | Role of promoter methylation in increased methionine adenosyltransferase 2A expression in human liver cancer. | 2001 | Am. J. Physiol. Gastrointest. Liver Physiol. | pmid:11208539 |
| Chen C et al. | Evidence that silencing of the HPRT promoter by DNA methylation is mediated by critical CpG sites. | 2001 | J. Biol. Chem. | pmid:11013250 |
| Ullerås E et al. | Inhibition of histone deacetylase activity causes cell type-specific induction of the PDGF-B promoter only in the absence of activation by its enhancer. | 2001 | Exp. Cell Res. | pmid:11640883 |
| Grandjean V et al. | Relationship between DNA methylation, histone H4 acetylation and gene expression in the mouse imprinted Igf2-H19 domain. | 2001 | FEBS Lett. | pmid:11163765 |
| Lizcano F et al. | Cell type-specific roles of histone deacetylase in TR ligand-independent transcriptional repression. | 2001 | Mol. Cell. Endocrinol. | pmid:11165035 |
| Nervi C et al. | Inhibition of histone deacetylase activity by trichostatin A modulates gene expression during mouse embryogenesis without apparent toxicity. | 2001 | Cancer Res. | pmid:11245412 |
| Zhu WG et al. | DNA methyltransferase inhibition enhances apoptosis induced by histone deacetylase inhibitors. | 2001 | Cancer Res. | pmid:11245429 |
| Muth V et al. | Acetylation of TAF(I)68, a subunit of TIF-IB/SL1, activates RNA polymerase I transcription. | 2001 | EMBO J. | pmid:11250901 |
| Laribee RN and Klemsz MJ | Loss of PU.1 expression following inhibition of histone deacetylases. | 2001 | J. Immunol. | pmid:11673528 |
| Biade S et al. | Chemical agents that promote chromatin compaction radiosensitize tumour cells. | 2001 | Int. J. Radiat. Biol. | pmid:11682008 |
| Mishra SK et al. | Dynamic chromatin remodeling on the HER2 promoter in human breast cancer cells. | 2001 | FEBS Lett. | pmid:11682064 |
| Rombouts K et al. | Trichostatin A, lead compound for development of antifibrogenic drugs. | 2001 Jul-Sep | Acta Gastroenterol. Belg. | pmid:11680040 |
| McGuinness MC et al. | Evaluation of pharmacological induction of fatty acid beta-oxidation in X-linked adrenoleukodystrophy. | 2001 Sep-Oct | Mol. Genet. Metab. | pmid:11592822 |
| Sawa H et al. | Histone deacetylase inhibitors such as sodium butyrate and trichostatin A inhibit vascular endothelial growth factor (VEGF) secretion from human glioblastoma cells. | 2002 | Brain Tumor Pathol | pmid:12622137 |
| Suzuki-Mizushima Y et al. | Enhancement of NGF- and cholera toxin-induced neurite outgrowth by butyrate in PC12 cells. | 2002 | Brain Res. | pmid:12270499 |
| Boutillier AL et al. | Constitutive repression of E2F1 transcriptional activity through HDAC proteins is essential for neuronal survival. | 2002 | Ann. N. Y. Acad. Sci. | pmid:12485907 |
| Zarnegar R et al. | Increasing the effectiveness of radioactive iodine therapy in the treatment of thyroid cancer using Trichostatin A, a histone deacetylase inhibitor. | 2002 | Surgery | pmid:12490845 |
| Valapour M et al. | Histone deacetylation inhibits IL4 gene expression in T cells. | 2002 | J. Allergy Clin. Immunol. | pmid:11842291 |
| McBurney MW et al. | Evidence for repeat-induced gene silencing in cultured Mammalian cells: inactivation of tandem repeats of transfected genes. | 2002 | Exp. Cell Res. | pmid:11855851 |
| Hou M et al. | The histone deacetylase inhibitor trichostatin A derepresses the telomerase reverse transcriptase (hTERT) gene in human cells. | 2002 | Exp. Cell Res. | pmid:11855854 |
| Remiszewski SW et al. | Inhibitors of human histone deacetylase: synthesis and enzyme and cellular activity of straight chain hydroxamates. | 2002 | J. Med. Chem. | pmid:11831887 |
| Avram D et al. | COUP-TF (chicken ovalbumin upstream promoter transcription factor)-interacting protein 1 (CTIP1) is a sequence-specific DNA binding protein. | 2002 | Biochem. J. | pmid:12196208 |
| Guo Y et al. | Regulation of DNA methylation in human breast cancer. Effect on the urokinase-type plasminogen activator gene production and tumor invasion. | 2002 | J. Biol. Chem. | pmid:12198113 |
| Wittich S et al. | Structure-activity relationships on phenylalanine-containing inhibitors of histone deacetylase: in vitro enzyme inhibition, induction of differentiation, and inhibition of proliferation in Friend leukemic cells. | 2002 | J. Med. Chem. | pmid:12109913 |
| Toyooka S et al. | Differential expression of FEZ1/LZTS1 gene in lung cancers and their cell cultures. | 2002 | Clin. Cancer Res. | pmid:12114433 |
| Choi HS et al. | Trichostatin A, a histone deacetylase inhibitor, activates the IGFBP-3 promoter by upregulating Sp1 activity in hepatoma cells: alteration of the Sp1/Sp3/HDAC1 multiprotein complex. | 2002 | Biochem. Biophys. Res. Commun. | pmid:12200149 |
| Maecker HL et al. | Epigenetic changes in tumor Fas levels determine immune escape and response to therapy. | 2002 | Cancer Cell | pmid:12204534 |
| Toyooka S et al. | Progressive aberrant methylation of the RASSF1A gene in simian virus 40 infected human mesothelial cells. | 2002 | Oncogene | pmid:12082623 |
| Maehara K et al. | Effects of histone acetylation on transcriptional regulation of manganese superoxide dismutase gene. | 2002 | Biochem. Biophys. Res. Commun. | pmid:12083788 |
| Iezzi S et al. | Stage-specific modulation of skeletal myogenesis by inhibitors of nuclear deacetylases. | 2002 | Proc. Natl. Acad. Sci. U.S.A. | pmid:12032356 |
| Nielsen J | Combinatorial synthesis of natural products. | 2002 | Curr Opin Chem Biol | pmid:12023109 |
| Mikkelsen IM et al. | The expression of gamma-glutamyltransferase in rat colon carcinoma cells is distinctly regulated during differentiation and oxidative stress. | 2002 | Mol. Cell. Biochem. | pmid:12030384 |