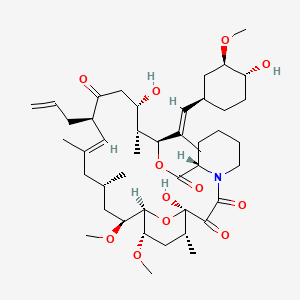
tacrolimus
Tacrolimus is a lipid of Polyketides (PK) class. Tacrolimus is associated with abnormalities such as Renal glomerular disease. The involved functions are known as inhibitors, Fungicidal activity, Metabolic Inhibition, Excretory function and Dephosphorylation. Tacrolimus often locates in Hepatic, Mitochondrial matrix and Inner mitochondrial membrane. The associated genes with Tacrolimus are RHOA gene and BGN gene.
Cross Reference
Introduction
To understand associated biological information of tacrolimus, we collected biological information of abnormalities, associated pathways, cellular/molecular locations, biological functions, related genes/proteins, lipids and common seen animal/experimental models with organized paragraphs from literatures.
What diseases are associated with tacrolimus?
tacrolimus is suspected in Renal glomerular disease, Candidiasis, Mycoses, PARKINSON DISEASE, LATE-ONSET, Morphologically altered structure, Skin Diseases, Infectious and other diseases in descending order of the highest number of associated sentences.
Related references are mostly published in these journals:
- Antimicrob. Agents Chemother. (2)
- Am. J. Physiol. Renal Physiol. (1)
- Drug Metab. Dispos. (1)
- Others (1)
| Disease | Cross reference | Weighted score | Related literature |
|---|
Possible diseases from mapped MeSH terms on references
We collected disease MeSH terms mapped to the references associated with tacrolimus
PubChem Associated disorders and diseases
What pathways are associated with tacrolimus
There are no associated biomedical information in the current reference collection.
PubChem Biomolecular Interactions and Pathways
Link to PubChem Biomolecular Interactions and PathwaysWhat cellular locations are associated with tacrolimus?
Visualization in cellular structure
Associated locations are in red color. Not associated locations are in black.
Related references are published most in these journals:
| Location | Cross reference | Weighted score | Related literatures |
|---|
What functions are associated with tacrolimus?
Related references are published most in these journals:
| Function | Cross reference | Weighted score | Related literatures |
|---|
What lipids are associated with tacrolimus?
Related references are published most in these journals:
| Lipid concept | Cross reference | Weighted score | Related literatures |
|---|
What genes are associated with tacrolimus?
Related references are published most in these journals:
| Gene | Cross reference | Weighted score | Related literatures |
|---|
What common seen animal models are associated with tacrolimus?
There are no associated biomedical information in the current reference collection.
NCBI Entrez Crosslinks
All references with tacrolimus
Download all related citations| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Spada M et al. | Randomized trial of basiliximab induction versus steroid therapy in pediatric liver allograft recipients under tacrolimus immunosuppression. | 2006 | Am. J. Transplant. | pmid:16771811 |
| Saliba F et al. | Efficacy and Safety of Everolimus and Mycophenolic Acid With Early Tacrolimus Withdrawal After Liver Transplantation: A Multicenter Randomized Trial. | 2017 | Am. J. Transplant. | pmid:28133906 |
| de Fijter JW et al. | Early Conversion From Calcineurin Inhibitor- to Everolimus-Based Therapy Following Kidney Transplantation: Results of the Randomized ELEVATE Trial. | 2017 | Am. J. Transplant. | pmid:28027625 |
| Singh K et al. | Superiority of rapamycin over tacrolimus in preserving nonhuman primate Treg half-life and phenotype after adoptive transfer. | 2014 | Am. J. Transplant. | pmid:25359003 |
| Dugast E et al. | Failure of Calcineurin Inhibitor (Tacrolimus) Weaning Randomized Trial in Long-Term Stable Kidney Transplant Recipients. | 2016 | Am. J. Transplant. | pmid:27367750 |
| Tang Q | Pharmacokinetics of therapeutic Tregs. | 2014 | Am. J. Transplant. | pmid:25358900 |
| Wen X et al. | Comparison of Utilization and Clinical Outcomes for Belatacept- and Tacrolimus-Based Immunosuppression in Renal Transplant Recipients. | 2016 | Am. J. Transplant. | pmid:27137884 |
| Trofe-Clark J et al. | Immunosuppression, generic drugs and the FDA. | 2012 | Am. J. Transplant. | pmid:22176626 |
| Sikma MA et al. | Pharmacokinetics and Toxicity of Tacrolimus Early After Heart and Lung Transplantation. | 2015 | Am. J. Transplant. | pmid:26053114 |
| Latran M | Response to Klintmalm on the use of generic immunosuppression. | 2012 | Am. J. Transplant. | pmid:22176636 |
| Ellis CL and Racusen LC | Mild rise in creatinine six months post kidney transplant. | 2012 | Am. J. Transplant. | pmid:22845913 |
| de Fontbrune FS et al. | Veno-occlusive disease of the liver after lung transplantation. | 2007 | Am. J. Transplant. | pmid:17697264 |
| Abdelmalek MF et al. | Sirolimus conversion regimen versus continued calcineurin inhibitors in liver allograft recipients: a randomized trial. | 2012 | Am. J. Transplant. | pmid:22233522 |
| Boudjema K et al. | Reduced-dose tacrolimus with mycophenolate mofetil vs. standard-dose tacrolimus in liver transplantation: a randomized study. | 2011 | Am. J. Transplant. | pmid:21466650 |
| Krenzien F et al. | Age-Dependent Metabolic and Immunosuppressive Effects of Tacrolimus. | 2017 | Am. J. Transplant. | pmid:27754593 |
| Gupta G et al. | Safe Conversion From Tacrolimus to Belatacept in High Immunologic Risk Kidney Transplant Recipients With Allograft Dysfunction. | 2015 | Am. J. Transplant. | pmid:25988397 |
| Saikali JA et al. | Sirolimus may promote thrombotic microangiopathy. | 2003 | Am. J. Transplant. | pmid:12603218 |
| Wang Q et al. | Biodegradable microsphere-loaded tacrolimus enhanced the effect on mice islet allograft and reduced the adverse effect on insulin secretion. | 2004 | Am. J. Transplant. | pmid:15084166 |
| Schubert M et al. | Pharmacokinetics of sirolimus and tacrolimus in pediatric transplant patients. | 2004 | Am. J. Transplant. | pmid:15084173 |
| ter Meulen CG et al. | Steroid-withdrawal at 3 days after renal transplantation with anti-IL-2 receptor alpha therapy: a prospective, randomized, multicenter study. | 2004 | Am. J. Transplant. | pmid:15084178 |
| Borrows R et al. | Steroid sparing with tacrolimus and mycophenolate mofetil in renal transplantation. | 2004 | Am. J. Transplant. | pmid:15476485 |
| Hernández-Fisac I et al. | Tacrolimus-induced diabetes in rats courses with suppressed insulin gene expression in pancreatic islets. | 2007 | Am. J. Transplant. | pmid:17725683 |
| Suszynski TM et al. | Prospective randomized trial of maintenance immunosuppression with rapid discontinuation of prednisone in adult kidney transplantation. | 2013 | Am. J. Transplant. | pmid:23432755 |
| Damon C et al. | Predictive Modeling of Tacrolimus Dose Requirement Based on High-Throughput Genetic Screening. | 2017 | Am. J. Transplant. | pmid:27597269 |
| Ferguson R et al. | Immunosuppression with belatacept-based, corticosteroid-avoiding regimens in de novo kidney transplant recipients. | 2011 | Am. J. Transplant. | pmid:21114656 |
| Shuker N et al. | A Randomized Controlled Trial Comparing the Efficacy of Cyp3a5 Genotype-Based With Body-Weight-Based Tacrolimus Dosing After Living Donor Kidney Transplantation. | 2016 | Am. J. Transplant. | pmid:26714287 |
| Frassetto LA and Benet LZ | Pharmacogenomics and transplantation: where are we? | 2004 | Am. J. Transplant. | pmid:15147415 |
| MacPhee IA et al. | The influence of pharmacogenetics on the time to achieve target tacrolimus concentrations after kidney transplantation. | 2004 | Am. J. Transplant. | pmid:15147425 |
| Artz MA et al. | Conversion from cyclosporine to tacrolimus improves quality-of-life indices, renal graft function and cardiovascular risk profile. | 2004 | Am. J. Transplant. | pmid:15147428 |
| Hirsch HH et al. | Polyomavirus BK replication in de novo kidney transplant patients receiving tacrolimus or cyclosporine: a prospective, randomized, multicenter study. | 2013 | Am. J. Transplant. | pmid:23137180 |
| Mujtaba MA et al. | Conversion from tacrolimus to belatacept to prevent the progression of chronic kidney disease in pancreas transplantation: case report of two patients. | 2014 | Am. J. Transplant. | pmid:25179306 |
| Koefoed-Nielsen PB et al. | Blood tacrolimus levels and calcineurin phosphatase activity early after renal transplantation. | 2002 | Am. J. Transplant. | pmid:12099520 |
| Klintmalm GB | Immunosuppression, generic drugs and the FDA. | 2011 | Am. J. Transplant. | pmid:21794082 |
| Böger CA et al. | Reverse diastolic intrarenal flow due to calcineurin inhibitor (CNI) toxicity. | 2006 | Am. J. Transplant. | pmid:16889550 |
| Shihab FS et al. | Effect of corticosteroid withdrawal on tacrolimus and mycophenolate mofetil exposure in a randomized multicenter study. | 2013 | Am. J. Transplant. | pmid:23167508 |
| Forns X and Navasa M | Cyclosporine A or tacrolimus for hepatitis C recurrence? An old debate. | 2011 | Am. J. Transplant. | pmid:21797970 |
| Tan HP et al. | Living donor renal transplantation using alemtuzumab induction and tacrolimus monotherapy. | 2006 | Am. J. Transplant. | pmid:16889606 |
| Alloway RR et al. | A randomized pharmacokinetic study of generic tacrolimus versus reference tacrolimus in kidney transplant recipients. | 2012 | Am. J. Transplant. | pmid:22759200 |
| Wlodarczyk Z et al. | Pharmacokinetics for once- versus twice-daily tacrolimus formulations in de novo kidney transplantation: a randomized, open-label trial. | 2009 | Am. J. Transplant. | pmid:19681813 |
| Hesselink DA et al. | Cyclosporine interacts with mycophenolic acid by inhibiting the multidrug resistance-associated protein 2. | 2005 | Am. J. Transplant. | pmid:15816878 |
| Chisholm-Burns MA et al. | Immunosuppressant therapy adherence and graft failure among pediatric renal transplant recipients. | 2009 | Am. J. Transplant. | pmid:19681814 |
| Byrne GW et al. | Warfarin or low-molecular-weight heparin therapy does not prolong pig-to-primate cardiac xenograft function. | 2005 | Am. J. Transplant. | pmid:15816881 |
| Schold JD and Kaplan B | AZA/tacrolimus is associated with similar outcomes as MMF/tacrolimus among renal transplant recipients. | 2009 | Am. J. Transplant. | pmid:19681827 |
| Lucey MR et al. | A comparison of tacrolimus and cyclosporine in liver transplantation: effects on renal function and cardiovascular risk status. | 2005 | Am. J. Transplant. | pmid:15816894 |
| Pillebout E et al. | Renal histopathological lesions after orthotopic liver transplantation (OLT). | 2005 | Am. J. Transplant. | pmid:15816895 |
| Arnold R et al. | Association between calcineurin inhibitor treatment and peripheral nerve dysfunction in renal transplant recipients. | 2013 | Am. J. Transplant. | pmid:23841745 |
| Vanrenterghem Y et al. | Minimization of immunosuppressive therapy after renal transplantation: results of a randomized controlled trial. | 2005 | Am. J. Transplant. | pmid:15636615 |
| Sindhi R et al. | Enhanced donor-specific alloreactivity occurs independently of immunosuppression in children with early liver rejection. | 2005 | Am. J. Transplant. | pmid:15636616 |
| Shergill AK et al. | Applicability, tolerability and efficacy of preemptive antiviral therapy in hepatitis C-infected patients undergoing liver transplantation. | 2005 | Am. J. Transplant. | pmid:15636619 |
| Tydén G et al. | ABO incompatible kidney transplantations without splenectomy, using antigen-specific immunoadsorption and rituximab. | 2005 | Am. J. Transplant. | pmid:15636623 |
| Woodle ES et al. | A multicenter pilot study of early (4-day) steroid cessation in renal transplant recipients under simulect, tacrolimus and sirolimus. | 2005 | Am. J. Transplant. | pmid:15636625 |
| Mian AN et al. | Mycoplasma hominis septic arthritis in a pediatric renal transplant recipient: case report and review of the literature. | 2005 | Am. J. Transplant. | pmid:15636628 |
| Gregoor PS and Weimar W | Tacrolimus and pure red-cell aplasia. | 2005 | Am. J. Transplant. | pmid:15636632 |
| Busque S et al. | Calcineurin-inhibitor-free immunosuppression based on the JAK inhibitor CP-690,550: a pilot study in de novo kidney allograft recipients. | 2009 | Am. J. Transplant. | pmid:19660021 |
| Gao R et al. | Effects of immunosuppressive drugs on in vitro neogenesis of human islets: mycophenolate mofetil inhibits the proliferation of ductal cells. | 2007 | Am. J. Transplant. | pmid:17391142 |
| Tremblay S et al. | A Steady-State Head-to-Head Pharmacokinetic Comparison of All FK-506 (Tacrolimus) Formulations (ASTCOFF): An Open-Label, Prospective, Randomized, Two-Arm, Three-Period Crossover Study. | 2017 | Am. J. Transplant. | pmid:27340950 |
| Padiyar A et al. | Clinical predictors of proteinuria after conversion to sirolimus in kidney transplant recipients. | 2010 | Am. J. Transplant. | pmid:20055793 |
| Hardinger KL et al. | BK-virus and the impact of pre-emptive immunosuppression reduction: 5-year results. | 2010 | Am. J. Transplant. | pmid:20055811 |
| Bhorade SM et al. | Comparison of three tacrolimus-based immunosuppressive regimens in lung transplantation. | 2003 | Am. J. Transplant. | pmid:14629288 |
| Pondrom S | The AJT report. | 2008 | Am. J. Transplant. | pmid:18324976 |
| De Simone P et al. | Everolimus with reduced tacrolimus in liver transplantation. | 2013 | Am. J. Transplant. | pmid:23601137 |
| Fan DM et al. | Successful ABO-incompatible living-related intestinal transplantation: a 2-year follow-up. | 2015 | Am. J. Transplant. | pmid:25808777 |
| Luther P and Baldwin D | Pioglitazone in the management of diabetes mellitus after transplantation. | 2004 | Am. J. Transplant. | pmid:15575920 |
| Cherukuri A et al. | An analysis of lymphocyte phenotype after steroid avoidance with either alemtuzumab or basiliximab induction in renal transplantation. | 2012 | Am. J. Transplant. | pmid:22390816 |
| Pascher A et al. | Protein kinase C inhibitor sotrastaurin in de novo liver transplant recipients: a randomized phase II trial. | 2015 | Am. J. Transplant. | pmid:25677074 |
| Chen G et al. | A synergistic effect between PG490-88 and tacrolimus prolongs renal allograft survival in monkeys. | 2006 | Am. J. Transplant. | pmid:16539628 |
| Okabayashi T et al. | Mobilization of host stem cells enables long-term liver transplant acceptance in a strongly rejecting rat strain combination. | 2011 | Am. J. Transplant. | pmid:21883903 |
| Hellemons ME et al. | Former smoking is a risk factor for chronic kidney disease after lung transplantation. | 2011 | Am. J. Transplant. | pmid:21883906 |
| Maes BD et al. | Differential effect of diarrhea on FK506 versus cyclosporine A trough levels and resultant prevention of allograft rejection in renal transplant recipients. | 2002 | Am. J. Transplant. | pmid:12484345 |
| Mazariegos GV et al. | Dendritic cell subset ratio in tolerant, weaning and non-tolerant liver recipients is not affected by extent of immunosuppression. | 2005 | Am. J. Transplant. | pmid:15643991 |
| Knechtle SJ et al. | Early and limited use of tacrolimus to avoid rejection in an alemtuzumab and sirolimus regimen for kidney transplantation: clinical results and immune monitoring. | 2009 | Am. J. Transplant. | pmid:19344431 |
| Bahra M et al. | MMF and calcineurin taper in recurrent hepatitis C after liver transplantation: impact on histological course. | 2005 | Am. J. Transplant. | pmid:15644002 |
| van Hooff JP et al. | Glucose metabolic disorder after transplantation. | 2007 | Am. J. Transplant. | pmid:17511670 |
| Min SI et al. | Conversion of twice-daily tacrolimus to once-daily tacrolimus formulation in stable pediatric kidney transplant recipients: pharmacokinetics and efficacy. | 2013 | Am. J. Transplant. | pmid:23734831 |
| Bunnapradist S et al. | Conversion from twice-daily tacrolimus to once-daily extended release tacrolimus (LCPT): the phase III randomized MELT trial. | 2013 | Am. J. Transplant. | pmid:23279614 |
| Ceschi A et al. | Acute calcineurin inhibitor overdose: analysis of cases reported to a national poison center between 1995 and 2011. | 2013 | Am. J. Transplant. | pmid:23279718 |
| Toso C et al. | Sequential kidney/islet transplantation: efficacy and safety assessment of a steroid-free immunosuppression protocol. | 2006 | Am. J. Transplant. | pmid:16611343 |
| Lacaille F et al. | Severe dysimmune cytopenia in children treated with tacrolimus after organ transplantation. | 2006 | Am. J. Transplant. | pmid:16611346 |
| Tedesco-Silva H et al. | Reduced Incidence of Cytomegalovirus Infection in Kidney Transplant Recipients Receiving Everolimus and Reduced Tacrolimus Doses. | 2015 | Am. J. Transplant. | pmid:25988935 |
| Pech T et al. | Intestinal regeneration, residual function and immunological priming following rescue therapy after rat small bowel transplantation. | 2012 | Am. J. Transplant. | pmid:22974463 |
| Kaplan B and Kirk AD | Tacrolimus and sirolimus: when bad things happen to good drugs. | 2006 | Am. J. Transplant. | pmid:16827845 |
| McAlister VC et al. | Cyclosporin versus tacrolimus as primary immunosuppressant after liver transplantation: a meta-analysis. | 2006 | Am. J. Transplant. | pmid:16827858 |
| Gallon L et al. | Long-term renal allograft function on a tacrolimus-based, pred-free maintenance immunosuppression comparing sirolimus vs. MMF. | 2006 | Am. J. Transplant. | pmid:16827862 |
| Grenda R et al. | A prospective, randomized, multicenter trial of tacrolimus-based therapy with or without basiliximab in pediatric renal transplantation. | 2006 | Am. J. Transplant. | pmid:16827869 |
| Vanhove T et al. | High Intrapatient Variability of Tacrolimus Concentrations Predicts Accelerated Progression of Chronic Histologic Lesions in Renal Recipients. | 2016 | Am. J. Transplant. | pmid:27013142 |
| Teuteberg JJ et al. | Alemtuzumab induction prior to cardiac transplantation with lower intensity maintenance immunosuppression: one-year outcomes. | 2010 | Am. J. Transplant. | pmid:19889126 |
| Haufroid V et al. | CYP3A5 and ABCB1 polymorphisms and tacrolimus pharmacokinetics in renal transplant candidates: guidelines from an experimental study. | 2006 | Am. J. Transplant. | pmid:17049058 |
| Shapiro R et al. | Alemtuzumab pre-conditioning with tacrolimus monotherapy in pediatric renal transplantation. | 2007 | Am. J. Transplant. | pmid:17908272 |
| Rush D et al. | Lack of benefit of early protocol biopsies in renal transplant patients receiving TAC and MMF: a randomized study. | 2007 | Am. J. Transplant. | pmid:17908280 |
| Turgut F and Akcay A | Renal function estimation in cirrhosis. | 2009 | Am. J. Transplant. | pmid:19519814 |
| Irish WD et al. | Cyclosporine versus tacrolimus treated liver transplant recipients with chronic hepatitis C: outcomes analysis of the UNOS/OPTN database. | 2011 | Am. J. Transplant. | pmid:21564522 |
| Tricot L et al. | Safety and efficacy of raltegravir in HIV-infected transplant patients cotreated with immunosuppressive drugs. | 2009 | Am. J. Transplant. | pmid:19519819 |
| Friman S et al. | Sotrastaurin, a novel small molecule inhibiting protein-kinase C: randomized phase II study in renal transplant recipients. | 2011 | Am. J. Transplant. | pmid:21564523 |
| Kofler S et al. | Proton pump inhibitors reduce mycophenolate exposure in heart transplant recipients-a prospective case-controlled study. | 2009 | Am. J. Transplant. | pmid:19519820 |
| Barbas AS et al. | Posterior reversible encephalopathy syndrome independently associated with tacrolimus and sirolimus after multivisceral transplantation. | 2013 | Am. J. Transplant. | pmid:23331705 |
| Gatault P et al. | Reduction of Extended-Release Tacrolimus Dose in Low-Immunological-Risk Kidney Transplant Recipients Increases Risk of Rejection and Appearance of Donor-Specific Antibodies: A Randomized Study. | 2017 | Am. J. Transplant. | pmid:27862923 |
| Fujishiro J et al. | Influence of immunosuppression on alloresponse, inflammation and contractile function of graft after intestinal transplantation. | 2010 | Am. J. Transplant. | pmid:20642681 |
| Martinez F et al. | High dose epoetin beta in the first weeks following renal transplantation and delayed graft function: Results of the Neo-PDGF Study. | 2010 | Am. J. Transplant. | pmid:20642691 |
| Miller LW | Cardiovascular toxicities of immunosuppressive agents. | 2002 | Am. J. Transplant. | pmid:12392286 |
| Brennan DC et al. | Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. | 2005 | Am. J. Transplant. | pmid:15707414 |