| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Imran A et al. | Lipid peroxidation diminishing perspective of isolated theaflavins and thearubigins from black tea in arginine induced renal malfunctional rats. | 2018 | Lipids Health Dis | pmid:30021615 |
| Ferreira-Nunes R et al. | Versatile chromatographic method for catechin determination in development of topical formulations containing natural extracts. | 2018 | Biomed. Chromatogr. | pmid:28809050 |
| Carbonaro M et al. | Human insulin fibrillogenesis in the presence of epigallocatechin gallate and melatonin: Structural insights from a biophysical approach. | 2018 | Int. J. Biol. Macromol. | pmid:29727655 |
| Wang YQ et al. | Suppressive Effects of EGCG on Cervical Cancer. | 2018 | Molecules | pmid:30213130 |
| BorutinskaitÄ— V et al. | Green tea polyphenol EGCG causes anti-cancerous epigenetic modulations in acute promyelocytic leukemia cells. | 2018 | Leuk. Lymphoma | pmid:28641467 |
| Luo L et al. | An approach for degradation of grape seed and skin proanthocyanidin polymers into oligomers by sulphurous acid. | 2018 | Food Chem | pmid:29606439 |
| Hori K et al. | Antioxidant phenolic compounds from the rhizomes of Astilbe rivularis. | 2018 | Nat. Prod. Res. | pmid:28361551 |
| Chaudhury S et al. | Probing the inhibitory potency of epigallocatechin gallate against human γB-crystallin aggregation: Spectroscopic, microscopic and simulation studies. | 2018 | Spectrochim Acta A Mol Biomol Spectrosc | pmid:29172128 |
| Chassagne F et al. | A metabolomic approach to identify anti-hepatocarcinogenic compounds from plants used traditionally in the treatment of liver diseases. | 2018 | Fitoterapia | pmid:29477305 |
| James KD et al. | Potential role of the mitochondria as a target for the hepatotoxic effects of (-)-epigallocatechin-3-gallate in mice. | 2018 | Food Chem. Toxicol. | pmid:29175576 |
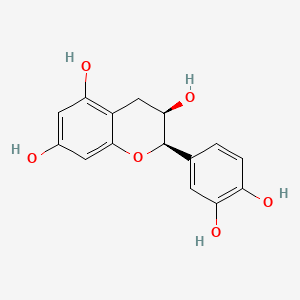
(-)-Epicatechin
(-)-Epicatechin is a lipid of Polyketides (PK) class.