| MeSH term | MeSH ID | Detail |
|---|---|---|
| Pancreatic Neoplasms | D010190 | 77 associated lipids |
| Mammary Neoplasms, Experimental | D008325 | 67 associated lipids |
| Lung Neoplasms | D008175 | 171 associated lipids |
| Liver Diseases | D008107 | 31 associated lipids |
| Hemolysis | D006461 | 131 associated lipids |
| Glioblastoma | D005909 | 27 associated lipids |
| Body Weight | D001835 | 333 associated lipids |
| Adenocarcinoma | D000230 | 166 associated lipids |
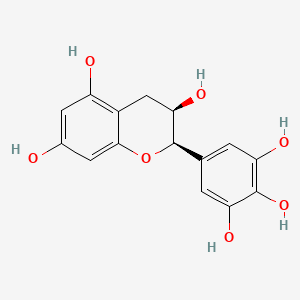
(-)-Epigallocatechin
(-)-Epigallocatechin is a lipid of Polyketides (PK) class. The involved functions are known as Protective Agents, inhibitors, Process, Drug Kinetics and Fermentation. (-)-epigallocatechin often locates in Hepatic, Blood, Membrane, Back and apical membrane. The associated genes with (-)-Epigallocatechin are ADRBK1 gene and FASTK Gene. The related lipids are 1,2-dilinolenoyl-3-(4-aminobutyryl)propane-1,2,3-triol. The related experimental models are Rodent Model and Transgenic Model.
Cross Reference
Introduction
To understand associated biological information of (-)-Epigallocatechin, we collected biological information of abnormalities, associated pathways, cellular/molecular locations, biological functions, related genes/proteins, lipids and common seen animal/experimental models with organized paragraphs from literatures.
What diseases are associated with (-)-Epigallocatechin?
There are no associated biomedical information in the current reference collection.
Possible diseases from mapped MeSH terms on references
We collected disease MeSH terms mapped to the references associated with (-)-Epigallocatechin
PubChem Associated disorders and diseases
What pathways are associated with (-)-Epigallocatechin
There are no associated biomedical information in the current reference collection.
PubChem Biomolecular Interactions and Pathways
Link to PubChem Biomolecular Interactions and PathwaysWhat cellular locations are associated with (-)-Epigallocatechin?
Visualization in cellular structure
Associated locations are in red color. Not associated locations are in black.
Related references are published most in these journals:
| Location | Cross reference | Weighted score | Related literatures |
|---|
What functions are associated with (-)-Epigallocatechin?
Related references are published most in these journals:
| Function | Cross reference | Weighted score | Related literatures |
|---|
What lipids are associated with (-)-Epigallocatechin?
Related references are published most in these journals:
| Lipid concept | Cross reference | Weighted score | Related literatures |
|---|
What genes are associated with (-)-Epigallocatechin?
Related references are published most in these journals:
| Gene | Cross reference | Weighted score | Related literatures |
|---|
What common seen animal models are associated with (-)-Epigallocatechin?
Rodent Model
Rodent Model are used in the study 'Dietary (-)-epicatechin as a potent inhibitor of βγ-secretase amyloid precursor protein processing.' (Cox CJ et al., 2015) and Rodent Model are used in the study 'Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals.' (Chow HH et al., 2005).
Transgenic Model
Transgenic Model are used in the study 'Dietary (-)-epicatechin as a potent inhibitor of βγ-secretase amyloid precursor protein processing.' (Cox CJ et al., 2015).
Related references are published most in these journals:
| Model | Cross reference | Weighted score | Related literatures |
|---|
NCBI Entrez Crosslinks
All references with (-)-Epigallocatechin
Download all related citations| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Yoon JH and Baek SJ | Molecular targets of dietary polyphenols with anti-inflammatory properties. | 2005 | Yonsei Med. J. | pmid:16259055 |
| Park YJ et al. | [6]-Gingerol induces cell cycle arrest and cell death of mutant p53-expressing pancreatic cancer cells. | 2006 | Yonsei Med. J. | pmid:17066513 |
| Park BJ et al. | In vitro antifungal activity of epigallocatechin 3-O-gallate against clinical isolates of dermatophytes. | 2011 | Yonsei Med. J. | pmid:21488200 |
| Kang MY et al. | Preventive effects of green tea (Camellia sinensis var. assamica) on diabetic nephropathy. | 2012 | Yonsei Med. J. | pmid:22187244 |
| Ikeda H et al. | [Mechanism of interaction between risperidone and tea catechin (2) influence of presence of galloyl group in catechin on insoluble complex formation with risperidone]. | 2012 | Yakugaku Zasshi | pmid:22214589 |
| Ikeda H et al. | [Mechanism of interaction between risperidone and tea catechin(1)complex formation of risperidone with epigallocatechin gallate]. | 2010 | Yakugaku Zasshi | pmid:21048420 |
| Zhu BT et al. | Rapid conversion of tea catechins to monomethylated products by rat liver cytosolic catechol-O-methyltransferase. | 2001 | Xenobiotica | pmid:11780762 |
| Van Amelsvoort JM et al. | Plasma concentrations of individual tea catechins after a single oral dose in humans. | 2001 | Xenobiotica | pmid:11780763 |
| Zhu M et al. | Pharmacokinetics and system linearity of tea catechins in rat. | 2001 | Xenobiotica | pmid:11334265 |
| Zhu BT et al. | Molecular modelling study of the mechanism of high-potency inhibition of human catechol-O-methyltransferase by (-)-epigallocatechin-3-O-gallate. | 2008 | Xenobiotica | pmid:18197555 |
| Javed T et al. | In-vitro antiviral activity of Solanum nigrum against Hepatitis C Virus. | 2011 | Virol. J. | pmid:21247464 |
| Jin UH et al. | Inhibitory effect of Salvia miltiorrhia BGE on matrix metalloproteinase-9 activity and migration of TNF-alpha-induced human aortic smooth muscle cells. | 2006 | Vascul. Pharmacol. | pmid:16540379 |
| Singh K et al. | Differential display mediated cloning of anthocyanidin reductase gene from tea (Camellia sinensis) and its relationship with the concentration of epicatechins. | 2009 | Tree Physiol. | pmid:19380395 |
| Nabekura T | Overcoming multidrug resistance in human cancer cells by natural compounds. | 2010 | Toxins (Basel) | pmid:22069634 |
| Russo M et al. | Phytochemicals in cancer prevention and therapy: truth or dare? | 2010 | Toxins (Basel) | pmid:22069598 |
| Yang C et al. | Caffeic acid phenethyl ester (CAPE) prevents transformation of human cells by arsenite (As) and suppresses growth of As-transformed cells. | 2005 | Toxicology | pmid:16085347 |
| Nishioka H et al. | Comparative efficacy of oligonol, catechin and (-)-epigallocatechin 3-O-gallate in modulating the potassium bromate-induced renal toxicity in rats. | 2006 | Toxicology | pmid:16916569 |
| Jeong JH et al. | Epigallocatechin 3-gallate attenuates neuronal damage induced by 3-hydroxykynurenine. | 2004 | Toxicology | pmid:14698567 |
| de MejÃa EG and RamÃrez-Mares MV | Leaf extract from Ardisia compressa protects against 1-nitropyrene-induced cytotoxicity and its antioxidant defense disruption in cultured rat hepatocytes. | 2002 | Toxicology | pmid:12204551 |
| Koh SH et al. | Epigallocatechin gallate prevents oxidative-stress-induced death of mutant Cu/Zn-superoxide dismutase (G93A) motoneuron cells by alteration of cell survival and death signals. | 2004 | Toxicology | pmid:15337584 |
| Fechtner S et al. | Molecular insights into the differences in anti-inflammatory activities of green tea catechins on IL-1β signaling in rheumatoid arthritis synovial fibroblasts. | 2017 | Toxicol. Appl. Pharmacol. | pmid:28532672 |
| Li G et al. | A tea catechin, epigallocatechin-3-gallate, is a unique modulator of the farnesoid X receptor. | 2012 | Toxicol. Appl. Pharmacol. | pmid:22178739 |
| Glei M et al. | Initial in vitro toxicity testing of functional foods rich in catechins and anthocyanins in human cells. | 2003 Oct-Dec | Toxicol In Vitro | pmid:14599469 |
| Babich H et al. | Differential in vitro cytotoxicity of (-)-epicatechin gallate (ECG) to cancer and normal cells from the human oral cavity. | 2005 | Toxicol In Vitro | pmid:15649637 |
| Yamauchi R et al. | Identification of epigallocatechin-3-gallate in green tea polyphenols as a potent inducer of p53-dependent apoptosis in the human lung cancer cell line A549. | 2009 | Toxicol In Vitro | pmid:19406223 |
| Mirasoli M et al. | Electronic nose and chiral-capillary electrophoresis in evaluation of the quality changes in commercial green tea leaves during a long-term storage. | 2014 | Talanta | pmid:25127562 |
| Olšovská J et al. | Ultra-high-performance liquid chromatography profiling method for chemical screening of proanthocyanidins in Czech hops. | 2013 | Talanta | pmid:24148495 |
| Nakazono M et al. | The chemiluminescence mechanism of 3,4-bis(3-indolyl)-1H-pyrrole-2,5-dione, and the characteristics of chemiluminescence developed in the reaction with CH(3)CNH(2)O(2)NaOH. | 2006 | Talanta | pmid:18970740 |
| Zuo Y et al. | Simultaneous determination of catechins, caffeine and gallic acids in green, Oolong, black and pu-erh teas using HPLC with a photodiode array detector. | 2002 | Talanta | pmid:18968631 |
| Liu J et al. | Borate complexation-assisted field-enhanced sample injection for on-line preconcentration of cis-diol-containing compounds in capillary electrophoresis. | 2009 | Talanta | pmid:19836518 |
| Castro J et al. | Determination of catechins and caffeine in proposed green tea standard reference materials by liquid chromatography-particle beam/electron ionization mass spectrometry (LC-PB/EIMS). | 2010 | Talanta | pmid:20875564 |
| Blaylock RL and Maroon J | Natural plant products and extracts that reduce immunoexcitotoxicity-associated neurodegeneration and promote repair within the central nervous system. | 2012 | Surg Neurol Int | pmid:22439110 |
| Petraglia AL et al. | Stuck at the bench: Potential natural neuroprotective compounds for concussion. | 2011 | Surg Neurol Int | pmid:22059141 |
| Wu X et al. | Analysis of binding interaction between (-)-epigallocatechin (EGC) and β-lactoglobulin by multi-spectroscopic method. | 2011 | Spectrochim Acta A Mol Biomol Spectrosc | pmid:21820944 |
| Roh C et al. | Label free inhibitor screening of hepatitis C virus (HCV) NS5B viral protein using RNA oligonucleotide. | 2011 | Sensors (Basel) | pmid:22163979 |
| Kobayashi Y et al. | Advanced taste sensors based on artificial lipids with global selectivity to basic taste qualities and high correlation to sensory scores. | 2010 | Sensors (Basel) | pmid:22319306 |
| Zhao C et al. | The galloyl catechins contributing to main antioxidant capacity of tea made from Camellia sinensis in China. | 2014 | ScientificWorldJournal | pmid:25243234 |
| de-Faria FM et al. | Antioxidant action of mangrove polyphenols against gastric damage induced by absolute ethanol and ischemia-reperfusion in the rat. | 2012 | ScientificWorldJournal | pmid:22654592 |
| Umar KM et al. | Engineering the production of major catechins by Escherichia coli carrying metabolite genes of Camellia sinensis. | 2012 | ScientificWorldJournal | pmid:22645428 |
| Sabhapondit S et al. | Diversity of catechin in northeast Indian tea cultivars. | 2012 | ScientificWorldJournal | pmid:22448135 |
| Qaâdan F et al. | Polyphenols from Ginkgo biloba. | 2010 Oct-Dec | Sci Pharm | pmid:21179324 |
| Di Paola R et al. | Green tea polyphenol extract attenuates lung injury in experimental model of carrageenan-induced pleurisy in mice. | 2005 | Respir. Res. | pmid:15987519 |
| Hazgui S et al. | Epigallocatechin-3-gallate (EGCG) inhibits the migratory behavior of tumor bronchial epithelial cells. | 2008 | Respir. Res. | pmid:18426555 |
| Plumb GW et al. | Antioxidant properties of gallocatechin and prodelphinidins from pomegranate peel. | 2002 | Redox Rep. | pmid:11981454 |
| Mizooku Y et al. | Analysis of oxidized epigallocatechin gallate by liquid chromatography/mass spectrometry. | 2003 | Rapid Commun. Mass Spectrom. | pmid:12876693 |
| Sang S et al. | Human urinary metabolite profile of tea polyphenols analyzed by liquid chromatography/electrospray ionization tandem mass spectrometry with data-dependent acquisition. | 2008 | Rapid Commun. Mass Spectrom. | pmid:18433082 |
| Bhatia N and Agarwal R | Detrimental effect of cancer preventive phytochemicals silymarin, genistein and epigallocatechin 3-gallate on epigenetic events in human prostate carcinoma DU145 cells. | 2001 | Prostate | pmid:11170137 |
| Thomas F et al. | Dihydrotestosterone sensitises LNCaP cells to death induced by epigallocatechin-3-Gallate (EGCG) or an IGF-I receptor inhibitor. | 2009 | Prostate | pmid:18942120 |
| Chang WC and Hsu FL | Inhibition of platelet activation and endothelial cell injury by flavan-3-ol and saikosaponin compounds. | 1991 | Prostaglandins Leukot. Essent. Fatty Acids | pmid:1946562 |
| Lotito SB and Fraga CG | Catechins delay lipid oxidation and alpha-tocopherol and beta-carotene depletion following ascorbate depletion in human plasma. | 2000 | Proc. Soc. Exp. Biol. Med. | pmid:10998196 |