| MeSH term | MeSH ID | Detail |
|---|---|---|
| Hemolysis | D006461 | 131 associated lipids |
| Stomach Ulcer | D013276 | 75 associated lipids |
| Diabetes Mellitus | D003920 | 90 associated lipids |
| Neoplasms, Experimental | D009374 | 10 associated lipids |
| Adenocarcinoma | D000230 | 166 associated lipids |
| Dermatitis, Contact | D003877 | 59 associated lipids |
| Pain | D010146 | 64 associated lipids |
| Lupus Erythematosus, Systemic | D008180 | 43 associated lipids |
| Lung Neoplasms | D008175 | 171 associated lipids |
| Pancreatic Neoplasms | D010190 | 77 associated lipids |
| Colonic Neoplasms | D003110 | 161 associated lipids |
| Diabetes Mellitus, Experimental | D003921 | 85 associated lipids |
| Mammary Neoplasms, Experimental | D008325 | 67 associated lipids |
| Body Weight | D001835 | 333 associated lipids |
| Edema | D004487 | 152 associated lipids |
| Prostatic Neoplasms | D011471 | 126 associated lipids |
| Osteosarcoma | D012516 | 50 associated lipids |
| Melanoma | D008545 | 69 associated lipids |
| Asthma | D001249 | 52 associated lipids |
| Glioma | D005910 | 112 associated lipids |
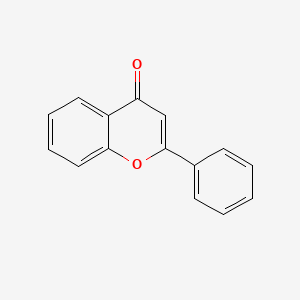
FLAVONE
FLAVONE is a lipid of Polyketides (PK) class. Flavone is associated with abnormalities such as Cardiovascular Diseases, Cerebrovascular accident, DERMATITIS HERPETIFORMIS, FAMILIAL, Hyperinsulinism and Inflammatory disorder. The involved functions are known as Oxidation-Reduction, Metabolic Inhibition, Inflammation, Phosphorylation and antioxidant activity. Flavone often locates in Endothelium, Hepatic, Protoplasm, Body tissue and Extracellular. The associated genes with FLAVONE are ICAM1 gene, BCL2L1 gene, MYC gene, TP53 gene and cytochrome c''. The related lipids are Promega, Steroids and Total cholesterol. The related experimental models are Knock-out, Disease model and Animal Disease Models.
Cross Reference
Introduction
To understand associated biological information of FLAVONE, we collected biological information of abnormalities, associated pathways, cellular/molecular locations, biological functions, related genes/proteins, lipids and common seen animal/experimental models with organized paragraphs from literatures.
What diseases are associated with FLAVONE?
FLAVONE is suspected in Diabetes Mellitus, Non-Insulin-Dependent, Obesity, Chronic Disease, Disintegration, Cardiovascular Diseases, Cerebrovascular accident and other diseases in descending order of the highest number of associated sentences.
Related references are mostly published in these journals:
| Disease | Cross reference | Weighted score | Related literature |
|---|
Possible diseases from mapped MeSH terms on references
We collected disease MeSH terms mapped to the references associated with FLAVONE
PubChem Associated disorders and diseases
What pathways are associated with FLAVONE
There are no associated biomedical information in the current reference collection.
PubChem Biomolecular Interactions and Pathways
Link to PubChem Biomolecular Interactions and PathwaysWhat cellular locations are associated with FLAVONE?
Visualization in cellular structure
Associated locations are in red color. Not associated locations are in black.
Related references are published most in these journals:
| Location | Cross reference | Weighted score | Related literatures |
|---|
What functions are associated with FLAVONE?
Related references are published most in these journals:
| Function | Cross reference | Weighted score | Related literatures |
|---|
What lipids are associated with FLAVONE?
Related references are published most in these journals:
| Lipid concept | Cross reference | Weighted score | Related literatures |
|---|
What genes are associated with FLAVONE?
Related references are published most in these journals:
| Gene | Cross reference | Weighted score | Related literatures |
|---|
What common seen animal models are associated with FLAVONE?
Knock-out
Knock-out are used in the study 'MATE2 mediates vacuolar sequestration of flavonoid glycosides and glycoside malonates in Medicago truncatula.' (Zhao J et al., 2011) and Knock-out are used in the study 'How can research on plants contribute to promoting human health?' (Martin C et al., 2011).
Disease model
Disease model are used in the study 'How can research on plants contribute to promoting human health?' (Martin C et al., 2011).
Animal Disease Models
Animal Disease Models are used in the study 'How can research on plants contribute to promoting human health?' (Martin C et al., 2011).
Related references are published most in these journals:
| Model | Cross reference | Weighted score | Related literatures |
|---|
NCBI Entrez Crosslinks
All references with FLAVONE
Download all related citations| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Galajda Z et al. | [Cardiovascular actions of a standardized polyphenol concentrate on patients undergoing coronary bypass grafting: a randomized, double-blind, placebo-controlled study]. | 2008 | Magy Seb | pmid:18504236 |
| March RE et al. | Empirically predicted 13C NMR chemical shifts for 8-hydroxyflavone starting from 7,8,4'-trihydroxyflavone and from 7,8-dihydroxyflavone. | 2008 | Magn Reson Chem | pmid:18389493 |
| Moon BH et al. | Complete assignment of (1)H and (13)C NMR data of dihydroxyflavone derivatives. | 2006 | Magn Reson Chem | pmid:16259053 |
| Burns DC et al. | A predictive tool for assessing (13)C NMR chemical shifts of flavonoids. | 2007 | Magn Reson Chem | pmid:17729229 |
| Bacher M et al. | Complete 1H and 13C NMR data assignment of new constituents from Severinia buxifolia. | 2010 | Magn Reson Chem | pmid:19937908 |
| Lin Z et al. | Enhanced electrochemiluminescence of Ru(bpy)3(2+) by flavone compounds. | 2008 Nov-Dec | Luminescence | pmid:18506909 |
| Berczyński P et al. | Synthesis and in vitro antioxidant activity study of some new piperazinyl flavone compounds. | 2017 | Luminescence | pmid:28569387 |
| Berczyński P et al. | Studies on the antioxidant activity of some thiazolidinedione, imidazolidinedione and rhodanine derivatives having a flavone core. | 2014 | Luminescence | pmid:24733694 |
| Yang Y et al. | Bioactivity-guided fractionation of the triglyceride-lowering component and in vivo and in vitro evaluation of hypolipidemic effects of Calyx seu Fructus Physalis. | 2012 | Lipids Health Dis | pmid:22413998 |
| Jung J et al. | Inhibition of estrogen action by 2-phenylchromone as AhR agonist in MCF-7 cells. | 2007 | Life Sci. | pmid:17950758 |
| Kuo SM | Transepithelial transport and accumulation of flavone in human intestinal Caco-2 cells. | 1998 | Life Sci. | pmid:9877222 |
| Zhai S et al. | Inhibition of methoxyresorufin demethylase activity by flavonoids in human liver microsomes. | 1998 | Life Sci. | pmid:9718089 |
| Hirano T et al. | Effects of synthetic and naturally occurring flavonoids on Na+,K+-ATPase: aspects of the structure-activity relationship and action mechanism. | 1989 | Life Sci. | pmid:2552245 |
| Ajay M et al. | Effects of flavonoids on vascular smooth muscle of the isolated rat thoracic aorta. | 2003 | Life Sci. | pmid:14623031 |
| Chen CC et al. | Calcium- and phosphatidylinositol 3-kinase/Akt-dependent activation of endothelial nitric oxide synthase by apigenin. | 2010 | Life Sci. | pmid:21034748 |
| Åania-Pietrzak B et al. | Modulation of MRP1 protein transport by plant, and synthetically modified flavonoids. | 2005 | Life Sci. | pmid:15916776 |
| Billard C et al. | Flavopiridol-induced iNOS downregulation during apoptosis of chronic lymphocytic leukemia cells is caspase-dependent. | 2008 | Leuk. Res. | pmid:17981326 |
| Quiney C et al. | Flavones and polyphenols inhibit the NO pathway during apoptosis of leukemia B-cells. | 2004 | Leuk. Res. | pmid:15203283 |
| Pepper C et al. | Flavopiridol induces apoptosis in B-cell chronic lymphocytic leukaemia cells through a p38 and ERK MAP kinase-dependent mechanism. | 2003 | Leuk. Lymphoma | pmid:12688354 |
| Wang XH et al. | Screen of micro-organisms for inducing the production of dragon's blood by leaf of Dracaena cochinchinensis. | 2010 | Lett. Appl. Microbiol. | pmid:20860774 |
| Rubin J et al. | Flavone-8-acetic acid inhibits ristocetin-induced platelet agglutination and prolongs bleeding time. | 1987 | Lancet | pmid:2889983 |
| Verweij J et al. | Rapid alkalinisation for flavone acetic acid administration: a potentially hazardous procedure. | 1988 | Lancet | pmid:2893206 |
| Davis HP et al. | Immune thrombocytopenia caused by flavone-8-acetic acid. | 1988 | Lancet | pmid:2893207 |
| Lee JM et al. | Acute Toxicity and General Pharmacological Action of QGC EXT. | 2012 | Korean J. Physiol. Pharmacol. | pmid:22416220 |
| Baek I et al. | Flavone Attenuates Vascular Contractions by Inhibiting RhoA/Rho Kinase Pathway. | 2009 | Korean J. Physiol. Pharmacol. | pmid:19885038 |
| Keller G and Leuenberger PM | [Aldose-reductase inhibitors and cataract formation (author's transl)]. | 1980 | Klin Monbl Augenheilkd | pmid:6775130 |
| Tamura M et al. | Effects of flavonoid compounds on the activity of NADPH diaphorase prepared from the mouse brain. | 1994 | Jpn. J. Pharmacol. | pmid:7990275 |
| Yamazoe Y et al. | Metabolic activation of pyrolysate arylamines by human liver microsomes; possible involvement of a P-488-H type cytochrome P-450. | 1988 | Jpn. J. Cancer Res. | pmid:3147272 |
| Paulin JJ et al. | Ultrastructural modifications during the metabolism of metronidazole by Trypanosoma cruzi. | 1983 | J. Submicrosc. Cytol. | pmid:6361276 |
| Collins-Burow BM et al. | Antiestrogenic activity of flavonoid phytochemicals mediated via the c-Jun N-terminal protein kinase pathway. Cell-type specific regulation of estrogen receptor alpha. | 2012 | J. Steroid Biochem. Mol. Biol. | pmid:22634477 |
| Saarinen N et al. | No evidence for the in vivo activity of aromatase-inhibiting flavonoids. | 2001 | J. Steroid Biochem. Mol. Biol. | pmid:11595503 |
| Ohno S et al. | Flavonoid inhibition of overexpressed human 3beta-hydroxysteroid dehydrogenase type II. | 2004 | J. Steroid Biochem. Mol. Biol. | pmid:15084349 |
| Ko JH et al. | Four glucosyltransferases from rice: cDNA cloning, expression, and characterization. | 2008 | J. Plant Physiol. | pmid:17363107 |
| Monici M et al. | Flavone photoreactivity. UV-induced reactions in organic solvents and micellar systems. | 1993 | J. Photochem. Photobiol. B, Biol. | pmid:8271117 |
| Anbazhagan V et al. | Investigations on the fluorescence quenching of 2,3-diazabicyclo[2.2.2]oct-2-ene by certain flavonoids. | 2008 | J. Photochem. Photobiol. B, Biol. | pmid:18440819 |
| Yao X et al. | Biochemical traits and proteomic changes in postharvest flowers of medicinal chrysanthemum exposed to enhanced UV-B radiation. | 2015 | J. Photochem. Photobiol. B, Biol. | pmid:26114222 |
| Yao X et al. | The changes in quality ingredients of Qi chrysanthemum flowers treated with elevated UV-B radiation at different growth stages. | 2015 | J. Photochem. Photobiol. B, Biol. | pmid:25792150 |
| Kim HP et al. | Anti-inflammatory plant flavonoids and cellular action mechanisms. | 2004 | J. Pharmacol. Sci. | pmid:15539763 |
| Enomoto R et al. | Wogonin prevents glucocorticoid-induced thymocyte apoptosis without diminishing its anti-inflammatory action. | 2007 | J. Pharmacol. Sci. | pmid:17690528 |
| Wootten D et al. | Modulation of the glucagon-like peptide-1 receptor signaling by naturally occurring and synthetic flavonoids. | 2011 | J. Pharmacol. Exp. Ther. | pmid:21075839 |
| BOHR DF et al. | The effects of various flavone glucosides on the rate of passage of Evans blue through the damaged capillary wall. | 1949 | J. Pharmacol. Exp. Ther. | pmid:15391936 |
| Lai MY et al. | Comparison of metabolic pharmacokinetics of baicalin and baicalein in rats. | 2003 | J. Pharm. Pharmacol. | pmid:12631413 |
| Bovicelli P et al. | Efficient synthesis of polyoxygenated flavones from naturally occurring flavanones. | 2007 | J. Pharm. Pharmacol. | pmid:18053332 |
| Sasaki K et al. | Effect of flavones on rat brain and lung matrix metalloproteinase activity measured by film in-situ zymography. | 2005 | J. Pharm. Pharmacol. | pmid:15831206 |
| Liu IX et al. | Baicalin synergy with beta-lactam antibiotics against methicillin-resistant Staphylococcus aureus and other beta-lactam-resistant strains of S. aureus. | 2000 | J. Pharm. Pharmacol. | pmid:10757427 |
| Chung MI et al. | Synthesis, antiplatelet and vasorelaxing effects of monooxygenated flavones and flavonoxypropanolamines. | 2001 | J. Pharm. Pharmacol. | pmid:11804390 |
| Lim SS et al. | Synthesis of flavonoids and their effects on aldose reductase and sorbitol accumulation in streptozotocin-induced diabetic rat tissues. | 2001 | J. Pharm. Pharmacol. | pmid:11370705 |
| Akao T et al. | Efflux of baicalin, a flavone glucuronide of Scutellariae Radix, on Caco-2 cells through multidrug resistance-associated protein 2. | 2007 | J. Pharm. Pharmacol. | pmid:17227625 |
| ANREP GV et al. | The spasmolytic action of flavone. | 1953 | J. Pharm. Pharmacol. | pmid:13035698 |
| Park H et al. | Anti-inflammatory activity of the synthetic C-C biflavonoids. | 2006 | J. Pharm. Pharmacol. | pmid:17331331 |