| MeSH term | MeSH ID | Detail |
|---|---|---|
| Hemolysis | D006461 | 131 associated lipids |
| Stomach Ulcer | D013276 | 75 associated lipids |
| Diabetes Mellitus | D003920 | 90 associated lipids |
| Neoplasms, Experimental | D009374 | 10 associated lipids |
| Adenocarcinoma | D000230 | 166 associated lipids |
| Dermatitis, Contact | D003877 | 59 associated lipids |
| Pain | D010146 | 64 associated lipids |
| Lupus Erythematosus, Systemic | D008180 | 43 associated lipids |
| Lung Neoplasms | D008175 | 171 associated lipids |
| Pancreatic Neoplasms | D010190 | 77 associated lipids |
| Colonic Neoplasms | D003110 | 161 associated lipids |
| Diabetes Mellitus, Experimental | D003921 | 85 associated lipids |
| Mammary Neoplasms, Experimental | D008325 | 67 associated lipids |
| Body Weight | D001835 | 333 associated lipids |
| Edema | D004487 | 152 associated lipids |
| Prostatic Neoplasms | D011471 | 126 associated lipids |
| Osteosarcoma | D012516 | 50 associated lipids |
| Melanoma | D008545 | 69 associated lipids |
| Asthma | D001249 | 52 associated lipids |
| Glioma | D005910 | 112 associated lipids |
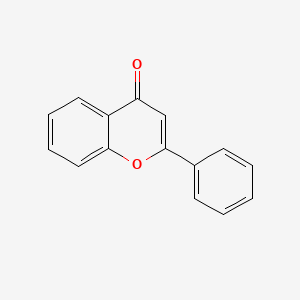
FLAVONE
FLAVONE is a lipid of Polyketides (PK) class. Flavone is associated with abnormalities such as Cardiovascular Diseases, Cerebrovascular accident, DERMATITIS HERPETIFORMIS, FAMILIAL, Hyperinsulinism and Inflammatory disorder. The involved functions are known as Oxidation-Reduction, Metabolic Inhibition, Inflammation, Phosphorylation and antioxidant activity. Flavone often locates in Endothelium, Hepatic, Protoplasm, Body tissue and Extracellular. The associated genes with FLAVONE are ICAM1 gene, BCL2L1 gene, MYC gene, TP53 gene and cytochrome c''. The related lipids are Promega, Steroids and Total cholesterol. The related experimental models are Knock-out, Disease model and Animal Disease Models.
Cross Reference
Introduction
To understand associated biological information of FLAVONE, we collected biological information of abnormalities, associated pathways, cellular/molecular locations, biological functions, related genes/proteins, lipids and common seen animal/experimental models with organized paragraphs from literatures.
What diseases are associated with FLAVONE?
FLAVONE is suspected in Diabetes Mellitus, Non-Insulin-Dependent, Obesity, Chronic Disease, Disintegration, Cardiovascular Diseases, Cerebrovascular accident and other diseases in descending order of the highest number of associated sentences.
Related references are mostly published in these journals:
| Disease | Cross reference | Weighted score | Related literature |
|---|
Possible diseases from mapped MeSH terms on references
We collected disease MeSH terms mapped to the references associated with FLAVONE
PubChem Associated disorders and diseases
What pathways are associated with FLAVONE
There are no associated biomedical information in the current reference collection.
PubChem Biomolecular Interactions and Pathways
Link to PubChem Biomolecular Interactions and PathwaysWhat cellular locations are associated with FLAVONE?
Visualization in cellular structure
Associated locations are in red color. Not associated locations are in black.
Related references are published most in these journals:
| Location | Cross reference | Weighted score | Related literatures |
|---|
What functions are associated with FLAVONE?
Related references are published most in these journals:
| Function | Cross reference | Weighted score | Related literatures |
|---|
What lipids are associated with FLAVONE?
Related references are published most in these journals:
| Lipid concept | Cross reference | Weighted score | Related literatures |
|---|
What genes are associated with FLAVONE?
Related references are published most in these journals:
| Gene | Cross reference | Weighted score | Related literatures |
|---|
What common seen animal models are associated with FLAVONE?
Knock-out
Knock-out are used in the study 'MATE2 mediates vacuolar sequestration of flavonoid glycosides and glycoside malonates in Medicago truncatula.' (Zhao J et al., 2011) and Knock-out are used in the study 'How can research on plants contribute to promoting human health?' (Martin C et al., 2011).
Disease model
Disease model are used in the study 'How can research on plants contribute to promoting human health?' (Martin C et al., 2011).
Animal Disease Models
Animal Disease Models are used in the study 'How can research on plants contribute to promoting human health?' (Martin C et al., 2011).
Related references are published most in these journals:
| Model | Cross reference | Weighted score | Related literatures |
|---|
NCBI Entrez Crosslinks
All references with FLAVONE
Download all related citations| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| LeJeune TM et al. | Mechanism of Action of Two Flavone Isomers Targeting Cancer Cells with Varying Cell Differentiation Status. | 2015 | PLoS ONE | pmid:26606169 |
| Jian J et al. | Bauhinia championii flavone inhibits apoptosis and autophagy via the PI3K/Akt pathway in myocardial ischemia/reperfusion injury in rats. | 2015 | Drug Des Devel Ther | pmid:26604691 |
| Real Hernandez LM et al. | Berry Phenolic Compounds Increase Expression of Hepatocyte Nuclear Factor-1α (HNF-1α) in Caco-2 and Normal Colon Cells Due to High Affinities with Transcription and Dimerization Domains of HNF-1α. | 2015 | PLoS ONE | pmid:26413797 |
| Boesten DM et al. | Protective Pleiotropic Effect of Flavonoids on NAD⺠Levels in Endothelial Cells Exposed to High Glucose. | 2015 | Oxid Med Cell Longev | pmid:26180598 |
| Liu B et al. | A step toward simplified detection of serum albumin on SDS-PAGE using an environment-sensitive flavone sensor. | 2015 | Chem. Commun. (Camb.) | pmid:26068596 |
| Hu J et al. | Genome-wide analysis of DNA methylation in photoperiod- and thermo-sensitive male sterile rice Peiai 64S. | 2015 | BMC Genomics | pmid:25887533 |
| Weng Z et al. | The novel flavone tetramethoxyluteolin is a potent inhibitor of human mast cells. | 2015 | J. Allergy Clin. Immunol. | pmid:25498791 |
| Lin CH et al. | Flavones inhibit breast cancer proliferation through the Akt/FOXO3a signaling pathway. | 2015 | BMC Cancer | pmid:26675309 |
| Nakamura K et al. | Effects of Hydroxy Groups in the A-Ring on the Anti-proteasome Activity of Flavone. | 2015 | Biol. Pharm. Bull. | pmid:25810454 |
| Aslan E and Adem S | In vitro effects of some flavones on human pyruvate kinase isoenzyme M2. | 2015 | J. Biochem. Mol. Toxicol. | pmid:25388478 |
| Imran S et al. | Synthesis of novel flavone hydrazones: in-vitro evaluation of α-glucosidase inhibition, QSAR analysis and docking studies. | 2015 | Eur J Med Chem | pmid:26491979 |
| Hao B et al. | The biosynthesis of allelopathic di-C-glycosylflavones from the roots of Desmodium incanum (G. Mey.) DC. | 2015 | Org. Biomol. Chem. | pmid:26478440 |
| Zhang F et al. | Forsythoneosides A-D, Neuroprotective Phenethanoid and Flavone Glycoside Heterodimers from the Fruits of Forsythia suspensa. | 2015 | J. Nat. Prod. | pmid:26422318 |
| Zhang M et al. | FlavonQ: an automated data processing tool for profiling flavone and flavonol glycosides with ultra-high-performance liquid chromatography-diode array detection-high resolution accurate mass-mass spectrometry. | 2015 | Anal. Chem. | pmid:26359695 |
| Fan Y et al. | The activation of Epimedium polysaccharide-propolis flavone liposome on Kupffer cells. | 2015 | Carbohydr Polym | pmid:26344320 |
| Zhao W et al. | Ammonium Iodide Induced Nonradical Regioselective Sulfenylation of Flavones via a C-H Functionalization Process. | 2015 | J. Org. Chem. | pmid:26291122 |
| Yan J et al. | The soybean R2R3 MYB transcription factor GmMYB100 negatively regulates plant flavonoid biosynthesis. | 2015 | Plant Mol. Biol. | pmid:26231207 |
| Yao X et al. | Biochemical traits and proteomic changes in postharvest flowers of medicinal chrysanthemum exposed to enhanced UV-B radiation. | 2015 | J. Photochem. Photobiol. B, Biol. | pmid:26114222 |
| Untergehrer M et al. | Structure-Dependent Deconjugation of Flavonoid Glucuronides by Human β-Glucuronidase - In Vitro and In Silico Analyses. | 2015 | Planta Med. | pmid:26018917 |
| Ting Y et al. | Viscoelastic Emulsion Improved the Bioaccessibility and Oral Bioavailability of Crystalline Compound: A Mechanistic Study Using in Vitro and in Vivo Models. | 2015 | Mol. Pharm. | pmid:25984595 |
| Xu S et al. | A novel flavone-based fluorescent probe for relay recognition of HSO3(-) and Al(3+). | 2015 | Spectrochim Acta A Mol Biomol Spectrosc | pmid:25965168 |
| Kim K et al. | Ru(II)-Catalyzed Site-Selective Hydroxylation of Flavone and Chromone Derivatives: The Importance of the 5-Hydroxyl Motif for the Inhibition of Aurora Kinases. | 2015 | Org. Lett. | pmid:25946086 |
| Benabdallah H and Gharzouli K | Effects of flavone on the contractile activity of the circular smooth muscle of the rabbit middle colon in vitro. | 2015 | Eur. J. Pharmacol. | pmid:25895637 |
| Li Q et al. | A combined strategy of mass fragmentation, post-column cobalt complexation and shift in ultraviolet absorption spectra to determine the uridine 5'-diphospho-glucuronosyltransferase metabolism profiling of flavones after oral administration of a flavone mixture in rats. | 2015 | J Chromatogr A | pmid:25892633 |
| Songsong W et al. | Characterization and rapid identification of chemical constituents of NaoXinTong capsules by UHPLC-linear ion trap/Orbitrap mass spectrometry. | 2015 | J Pharm Biomed Anal | pmid:25880241 |
| Yao X et al. | The changes in quality ingredients of Qi chrysanthemum flowers treated with elevated UV-B radiation at different growth stages. | 2015 | J. Photochem. Photobiol. B, Biol. | pmid:25792150 |
| Wang RL et al. | A novel cytochrome P450 CYP6AB14 gene in Spodoptera litura (Lepidoptera: Noctuidae) and its potential role in plant allelochemical detoxification. | 2015 | J. Insect Physiol. | pmid:25783953 |
| Zhu W et al. | Flavone inhibits nitric oxide synthase (NOS) activity, nitric oxide production and protein S-nitrosylation in breast cancer cells. | 2015 | Biochem. Biophys. Res. Commun. | pmid:25680459 |
| Zheng ZP et al. | Preparation, characterization, and preliminary antibrowning evaluations of norartocarpetin microemulsions. | 2015 | J. Agric. Food Chem. | pmid:25603116 |
| Ting Y et al. | Influence of processing parameters on morphology of polymethoxyflavone in emulsions. | 2015 | J. Agric. Food Chem. | pmid:25537008 |
| Berim A et al. | Identification of a unique 2-oxoglutarate-dependent flavone 7-O-demethylase completes the elucidation of the lipophilic flavone network in basil. | 2015 | Plant Cell Physiol. | pmid:25378691 |
| Kammalla AK et al. | Comparative pharmacokinetic interactions of Quercetin and Rutin in rats after oral administration of European patented formulation containing Hipphophae rhamnoides and Co-administration of Quercetin and Rutin. | 2015 | Eur J Drug Metab Pharmacokinet | pmid:24888486 |
| Almeida JR et al. | 3,6-Dimethoxy-6″,6″-Dimethyl-(7,8,2″,3″)-Chromeneflavone, a Flavonoid Isolated from Lonchocarpus Araripensis Benth. (Fabaceae), Reduces Nociceptive Behaviour in Mice. | 2015 | Phytother Res | pmid:26172339 |
| Hong H et al. | Human sex hormone-binding globulin binding affinities of 125 structurally diverse chemicals and comparison with their binding to androgen receptor, estrogen receptor, and α-fetoprotein. | 2015 | Toxicol. Sci. | pmid:25349334 |
| Zhang C et al. | Common and unique cis-acting elements mediate xanthotoxin and flavone induction of the generalist P450 CYP321A1. | 2014 | Sci Rep | pmid:25262756 |
| Berim A et al. | Unexpected roles for ancient proteins: flavone 8-hydroxylase in sweet basil trichomes is a Rieske-type, PAO-family oxygenase. | 2014 | Plant J. | pmid:25139498 |
| Goyal N et al. | Ethynylflavones, highly potent, and selective inhibitors of cytochrome P450 1A1. | 2014 | Chem. Res. Toxicol. | pmid:25033111 |
| Prasad VG et al. | Iminoflavones combat 1,2-dimethyl hydrazine-induced aberrant crypt foci development in colon cancer. | 2014 | Biomed Res Int | pmid:24995310 |
| Li LZ et al. | Flavonoids and other constituents from Aletris spicata and their chemotaxonomic significance. | 2014 | Nat. Prod. Res. | pmid:24896299 |
| Barreca D et al. | First evidence of C- and O-glycosyl flavone in blood orange (Citrus sinensis (L.) Osbeck) juice and their influence on antioxidant properties. | 2014 | Food Chem | pmid:24295703 |
| Zhang H et al. | Stimulatory effect of nobiletin, a citrus polymethoxy flavone, on catecholamine synthesis through Ser19 and Ser40 phosphorylation of tyrosine hydroxylase in cultured bovine adrenal medullary cells. | 2014 | Naunyn Schmiedebergs Arch. Pharmacol. | pmid:24043291 |
| Chen LX et al. | A new flavonoid from the aerial parts of Andrographis paniculata. | 2014 | Nat. Prod. Res. | pmid:24236635 |
| Zhao HN et al. | [A new flavone C-glycoside from leaves of Lophatherum gracile]. | 2014 | Zhongguo Zhong Yao Za Zhi | pmid:24761639 |
| Chen X et al. | Effects of epimedium polysaccharide-propolis flavone oral liquid on mucosal immunity in chickens. | 2014 | Int. J. Biol. Macromol. | pmid:24296407 |
| He R et al. | [Chemical constituents of leaves of Panax japonicus var. major]. | 2014 | Zhongguo Zhong Yao Za Zhi | pmid:25095375 |
| Król-Kogus B et al. | Application of one- and two-dimensional high-performance liquid chromatography methodologies for the analysis of C-glycosylflavones from fenugreek seeds. | 2014 | J Chromatogr A | pmid:25282311 |
| Feng XT et al. | Pollen Typhae total flavone improves insulin resistance in high-fat diet and low-dose streptozotocin-induced type 2 diabetic rats. | 2014 | Biosci. Biotechnol. Biochem. | pmid:25273139 |
| Wang MH et al. | A new antioxidant flavone glycoside from Scutellaria baicalensis Georgi. | 2014 | Nat. Prod. Res. | pmid:24995563 |
| Ahmed N et al. | Design, synthesis and antiproliferative activity of functionalized flavone-triazole-tetrahydropyran conjugates against human cancer cell lines. | 2014 | Eur J Med Chem | pmid:24941129 |
| Raghav N and Garg S | SAR studies of o-hydroxychalcones and their cyclized analogs and study them as novel inhibitors of cathepsin B and cathepsin H. | 2014 | Eur J Pharm Sci | pmid:24780403 |