| MeSH term | MeSH ID | Detail |
|---|---|---|
| Adenocarcinoma | D000230 | 166 associated lipids |
| Adenoma | D000236 | 40 associated lipids |
| Asthma | D001249 | 52 associated lipids |
| Body Weight | D001835 | 333 associated lipids |
| Carcinoma, Non-Small-Cell Lung | D002289 | 72 associated lipids |
| Cell Transformation, Neoplastic | D002471 | 126 associated lipids |
| Colonic Neoplasms | D003110 | 161 associated lipids |
| Cystitis | D003556 | 23 associated lipids |
| Dermatitis, Contact | D003877 | 59 associated lipids |
| Diabetes Mellitus | D003920 | 90 associated lipids |
| Diabetes Mellitus, Experimental | D003921 | 85 associated lipids |
| Edema | D004487 | 152 associated lipids |
| Encephalitis | D004660 | 15 associated lipids |
| Glioma | D005910 | 112 associated lipids |
| Hemolysis | D006461 | 131 associated lipids |
| Carcinoma, Hepatocellular | D006528 | 140 associated lipids |
| Hyperlipidemias | D006949 | 73 associated lipids |
| Hypertension | D006973 | 115 associated lipids |
| Leukemia P388 | D007941 | 43 associated lipids |
| Lung Neoplasms | D008175 | 171 associated lipids |
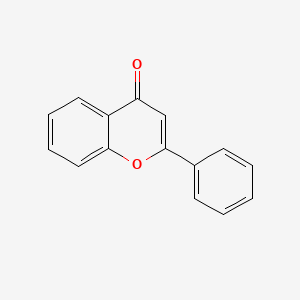
FLAVONE
FLAVONE is a lipid of Polyketides (PK) class. Flavone is associated with abnormalities such as Cardiovascular Diseases, Cerebrovascular accident, DERMATITIS HERPETIFORMIS, FAMILIAL, Hyperinsulinism and Inflammatory disorder. The involved functions are known as Oxidation-Reduction, Metabolic Inhibition, Inflammation, Phosphorylation and antioxidant activity. Flavone often locates in Endothelium, Hepatic, Protoplasm, Body tissue and Extracellular. The associated genes with FLAVONE are ICAM1 gene, BCL2L1 gene, MYC gene, TP53 gene and cytochrome c''. The related lipids are Promega, Steroids and Total cholesterol. The related experimental models are Knock-out, Disease model and Animal Disease Models.
Cross Reference
Introduction
To understand associated biological information of FLAVONE, we collected biological information of abnormalities, associated pathways, cellular/molecular locations, biological functions, related genes/proteins, lipids and common seen animal/experimental models with organized paragraphs from literatures.
What diseases are associated with FLAVONE?
FLAVONE is suspected in Diabetes Mellitus, Non-Insulin-Dependent, Obesity, Chronic Disease, Disintegration, Cardiovascular Diseases, Cerebrovascular accident and other diseases in descending order of the highest number of associated sentences.
Related references are mostly published in these journals:
| Disease | Cross reference | Weighted score | Related literature |
|---|
Possible diseases from mapped MeSH terms on references
We collected disease MeSH terms mapped to the references associated with FLAVONE
PubChem Associated disorders and diseases
What pathways are associated with FLAVONE
There are no associated biomedical information in the current reference collection.
PubChem Biomolecular Interactions and Pathways
Link to PubChem Biomolecular Interactions and PathwaysWhat cellular locations are associated with FLAVONE?
Visualization in cellular structure
Associated locations are in red color. Not associated locations are in black.
Related references are published most in these journals:
| Location | Cross reference | Weighted score | Related literatures |
|---|
What functions are associated with FLAVONE?
Related references are published most in these journals:
| Function | Cross reference | Weighted score | Related literatures |
|---|
What lipids are associated with FLAVONE?
Related references are published most in these journals:
| Lipid concept | Cross reference | Weighted score | Related literatures |
|---|
What genes are associated with FLAVONE?
Related references are published most in these journals:
| Gene | Cross reference | Weighted score | Related literatures |
|---|
What common seen animal models are associated with FLAVONE?
Knock-out
Knock-out are used in the study 'MATE2 mediates vacuolar sequestration of flavonoid glycosides and glycoside malonates in Medicago truncatula.' (Zhao J et al., 2011) and Knock-out are used in the study 'How can research on plants contribute to promoting human health?' (Martin C et al., 2011).
Disease model
Disease model are used in the study 'How can research on plants contribute to promoting human health?' (Martin C et al., 2011).
Animal Disease Models
Animal Disease Models are used in the study 'How can research on plants contribute to promoting human health?' (Martin C et al., 2011).
Related references are published most in these journals:
| Model | Cross reference | Weighted score | Related literatures |
|---|
NCBI Entrez Crosslinks
All references with FLAVONE
Download all related citations| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Kanho H et al. | Biotransformation of benzaldehyde-type and acetophenone-type derivatives by Pharbitis nil hairy roots. | 2005 | Chem. Pharm. Bull. | pmid:15802832 |
| Yoon JH and Baek SJ | Molecular targets of dietary polyphenols with anti-inflammatory properties. | 2005 | Yonsei Med. J. | pmid:16259055 |
| Wang TC et al. | Synthesis, and cytotoxic and antiplatelet activities of oxime- and methyloxime-containing flavone, isoflavone, and xanthone derivatives. | 2005 | Chem. Biodivers. | pmid:17191978 |
| Horvath CR et al. | Identification and quantification of eight flavones in root and shoot tissues of the medicinal plant huang-qin (Scutellaria baicalensis Georgi) using high-performance liquid chromatography with diode array and mass spectrometric detection. | 2005 | J Chromatogr A | pmid:15679157 |
| Cappelli C et al. | Computational study of conformational and chiroptical properties of (2R,3S,4R)-(+)-3,3',4,4',7-flavanpentol. | 2005 | Chirality | pmid:16196026 |
| Martens S and Mithöfer A | Flavones and flavone synthases. | 2005 | Phytochemistry | pmid:16137727 |
| Wenzel U et al. | Increased mitochondrial palmitoylcarnitine/carnitine countertransport by flavone causes oxidative stress and apoptosis in colon cancer cells. | 2005 | Cell. Mol. Life Sci. | pmid:16314920 |
| Cong Y et al. | A new flavonoside from Leonurus heterophyllus. | 2005 | J Asian Nat Prod Res | pmid:15621638 |
| Singh RP et al. | Acacetin inhibits cell growth and cell cycle progression, and induces apoptosis in human prostate cancer cells: structure-activity relationship with linarin and linarin acetate. | 2005 | Carcinogenesis | pmid:15637089 |
| Melidou M et al. | Protection against nuclear DNA damage offered by flavonoids in cells exposed to hydrogen peroxide: the role of iron chelation. | 2005 | Free Radic. Biol. Med. | pmid:16298684 |
| Ono M et al. | Radioiodinated flavones for in vivo imaging of beta-amyloid plaques in the brain. | 2005 | J. Med. Chem. | pmid:16279784 |
| Wang F et al. | Neuroactive flavonoids interacting with GABAA receptor complex. | 2005 | Curr Drug Targets CNS Neurol Disord | pmid:16266290 |
| Kim H et al. | Effects of naturally occurring compounds on fibril formation and oxidative stress of beta-amyloid. | 2005 | J. Agric. Food Chem. | pmid:16248550 |
| Kovacic P | Fundamental, electron transfer mechanism by pyrylium-type ions for the anticancer drugs 5,6-dimethylxanthenone-4-acetic acid (DMXAA) and flavone-8-acetic acid (FAA). | 2005 | Curr Med Chem Anticancer Agents | pmid:16178775 |
| Ozbey S et al. | Antibacterial 4-amino-N-(5-methylisoxazol-3-yl)-N-[(4-oxo-2-phenyl-4H-1-benzopyran-6-yl)methyl]benzenesulfonamide. | 2005 | Acta Crystallogr C | pmid:16143780 |
| Huong DT et al. | A new flavone and cytotoxic activity of flavonoid constituents isolated from Miliusa balansae (Annonaceae). | 2005 | Pharmazie | pmid:16124409 |
| Woodman OL et al. | Vasorelaxant and antioxidant activity of flavonols and flavones: structure-activity relationships. | 2005 | J. Cardiovasc. Pharmacol. | pmid:16116335 |
| Shiono M et al. | Phytochemistry: structure of the blue cornflower pigment. | 2005 | Nature | pmid:16094358 |
| Han Y | Ginkgo terpene component has an anti-inflammatory effect on Candida albicans-caused arthritic inflammation. | 2005 | Int. Immunopharmacol. | pmid:15829420 |
| Sawada Y and Ayabe S | Multiple mutagenesis of P450 isoflavonoid synthase reveals a key active-site residue. | 2005 | Biochem. Biophys. Res. Commun. | pmid:15809082 |
| Malolanarasimhan K et al. | Synthesis and biological study of a flavone acetic acid analogue containing an azido reporting group designed as a multifunctional binding site probe. | 2005 | Bioorg. Med. Chem. | pmid:15781383 |
| Ngadjui BT et al. | Prenylated chalcones, flavone and other constituents of the twigs of Dorstenia angusticornis and Dorstenia barteri var. subtriangularis. | 2005 | Phytochemistry | pmid:15771891 |
| Zhang J and Brodbelt JS | Silver complexation and tandem mass spectrometry for differentiation of isomeric flavonoid diglycosides. | 2005 | Anal. Chem. | pmid:15762583 |
| Göker H et al. | Synthesis and potent antimicrobial activity of some novel 2-phenyl or methyl-4H-1-benzopyran-4-ones carrying amidinobenzimidazoles. | 2005 | Bioorg. Med. Chem. | pmid:15698788 |
| Schütz K et al. | Characterization of phenolic acids and flavonoids in dandelion (Taraxacum officinale WEB. ex WIGG.) root and herb by high-performance liquid chromatography/electrospray ionization mass spectrometry. | 2005 | Rapid Commun. Mass Spectrom. | pmid:15593267 |
| Nagai S et al. | Kinetic study of the quenching reaction of singlet oxygen by flavonoids in ethanol solution. | 2005 | J Phys Chem B | pmid:16851486 |
| Likhitwitayawuid K et al. | Phenolics with antiviral activity from Millettia erythrocalyx and Artocarpus lakoocha. | 2005 | Nat. Prod. Res. | pmid:15715263 |
| Furuya H et al. | Some flavonoids and DHEA-S prevent the cis-effect of expanded CTG repeats in a stable PC12 cell transformant. | 2005 | Biochem. Pharmacol. | pmid:15652241 |
| Wenzig E et al. | Flavonolignans from Avena sativa. | 2005 | J. Nat. Prod. | pmid:15730266 |
| Cai H et al. | The rice bran constituent tricin potently inhibits cyclooxygenase enzymes and interferes with intestinal carcinogenesis in ApcMin mice. | 2005 | Mol. Cancer Ther. | pmid:16170019 |
| Takeda K et al. | Components of protocyanin, a blue pigment from the blue flowers of Centaurea cyanus. | 2005 | Phytochemistry | pmid:16005311 |
| Garritano S et al. | Assessment of estrogenic activity of flavonoids from Mediterranean plants using an in vitro short-term test. | 2005 | Phytomedicine | pmid:15693722 |
| Girish KS and Kemparaju K | Inhibition of Naja naja venom hyaluronidase by plant-derived bioactive components and polysaccharides. | 2005 | Biochemistry Mosc. | pmid:16212553 |
| Ahmed-Belkacem A et al. | Flavonoid structure-activity studies identify 6-prenylchrysin and tectochrysin as potent and specific inhibitors of breast cancer resistance protein ABCG2. | 2005 | Cancer Res. | pmid:15930306 |
| UydeÅŸ-DoÄŸan BS et al. | The comparison of the relaxant effects of two methoxylated flavones in rat aortic rings. | 2005 | Vascul. Pharmacol. | pmid:16169284 |
| Chi YS and Kim HP | Suppression of cyclooxygenase-2 expression of skin fibroblasts by wogonin, a plant flavone from Scutellaria radix. | 2005 | Prostaglandins Leukot. Essent. Fatty Acids | pmid:15589400 |
| Lu H et al. | Crystal structure of a human cyclin-dependent kinase 6 complex with a flavonol inhibitor, fisetin. | 2005 | J. Med. Chem. | pmid:15689157 |
| Capodice JL et al. | Complementary and alternative medicine for chronic prostatitis/chronic pelvic pain syndrome. | 2005 | Evid Based Complement Alternat Med | pmid:16322807 |
| Xie F et al. | The osteoprotective effect of Herba epimedii (HEP) extract in vivo and in vitro. | 2005 | Evid Based Complement Alternat Med | pmid:16136213 |
| Carrara M et al. | Mono- or di-fluorinated analogues of flavone-8-acetic acid: synthesis and in vitro biological activity. | 2005 Mar-Apr | Anticancer Res. | pmid:15868960 |
| Bas E et al. | Anti-inflammatory activity of 5-O-demethylnobiletin, a polymethoxyflavone isolated from Sideritis tragoriganum. | 2006 | Planta Med. | pmid:16491449 |
| Tsuji PA et al. | Accumulation and metabolism of the anticancer flavonoid 5,7-dimethoxyflavone compared to its unmethylated analog chrysin in the Atlantic killifish. | 2006 | Chem. Biol. Interact. | pmid:16999945 |
| Caddick S et al. | Synthetic ligands for apo-neocarzinostatin. | 2006 | J. Am. Chem. Soc. | pmid:16568976 |
| Qi S et al. | Simultaneous determination of bioactive flavone derivatives in Chinese herb extraction by capillary electrophoresis used different electrolyte systems--borate and ionic liquids. | 2006 | J Chromatogr A | pmid:16464459 |
| Nguyen MT et al. | Xanthine oxidase inhibitors from the flowers of Chrysanthemum sinense. | 2006 | Planta Med. | pmid:16450295 |
| Sandabe UK et al. | Phytochemical screening and effect of aqueous extract of Ficus sycomorus L. (Moraceae) stem bark on muscular activity in laboratory animals. | 2006 | J Ethnopharmacol | pmid:16448793 |
| Hamada M et al. | TCDD-induced CYP1A1 expression, an index of dioxin toxicity, is suppressed by flavonoids permeating the human intestinal Caco-2 cell monolayers. | 2006 | J. Agric. Food Chem. | pmid:17090139 |
| Capasso R et al. | Inhibition of rat vas deferens contractions by flavonoids in-vitro. | 2006 | J. Pharm. Pharmacol. | pmid:16536906 |
| Li H et al. | Prediction of estrogen receptor agonists and characterization of associated molecular descriptors by statistical learning methods. | 2006 | J. Mol. Graph. Model. | pmid:16497524 |
| Opassiri R et al. | Analysis of rice glycosyl hydrolase family 1 and expression of Os4bglu12 beta-glucosidase. | 2006 | BMC Plant Biol. | pmid:17196101 |