| MeSH term | MeSH ID | Detail |
|---|---|---|
| Adenocarcinoma | D000230 | 166 associated lipids |
| Adenoma | D000236 | 40 associated lipids |
| Asthma | D001249 | 52 associated lipids |
| Body Weight | D001835 | 333 associated lipids |
| Carcinoma, Non-Small-Cell Lung | D002289 | 72 associated lipids |
| Cell Transformation, Neoplastic | D002471 | 126 associated lipids |
| Colonic Neoplasms | D003110 | 161 associated lipids |
| Cystitis | D003556 | 23 associated lipids |
| Dermatitis, Contact | D003877 | 59 associated lipids |
| Diabetes Mellitus | D003920 | 90 associated lipids |
| Diabetes Mellitus, Experimental | D003921 | 85 associated lipids |
| Edema | D004487 | 152 associated lipids |
| Encephalitis | D004660 | 15 associated lipids |
| Glioma | D005910 | 112 associated lipids |
| Hemolysis | D006461 | 131 associated lipids |
| Carcinoma, Hepatocellular | D006528 | 140 associated lipids |
| Hyperlipidemias | D006949 | 73 associated lipids |
| Hypertension | D006973 | 115 associated lipids |
| Leukemia P388 | D007941 | 43 associated lipids |
| Lung Neoplasms | D008175 | 171 associated lipids |
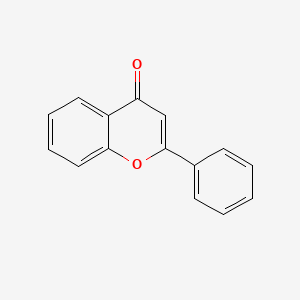
FLAVONE
FLAVONE is a lipid of Polyketides (PK) class. Flavone is associated with abnormalities such as Cardiovascular Diseases, Cerebrovascular accident, DERMATITIS HERPETIFORMIS, FAMILIAL, Hyperinsulinism and Inflammatory disorder. The involved functions are known as Oxidation-Reduction, Metabolic Inhibition, Inflammation, Phosphorylation and antioxidant activity. Flavone often locates in Endothelium, Hepatic, Protoplasm, Body tissue and Extracellular. The associated genes with FLAVONE are ICAM1 gene, BCL2L1 gene, MYC gene, TP53 gene and cytochrome c''. The related lipids are Promega, Steroids and Total cholesterol. The related experimental models are Knock-out, Disease model and Animal Disease Models.
Cross Reference
Introduction
To understand associated biological information of FLAVONE, we collected biological information of abnormalities, associated pathways, cellular/molecular locations, biological functions, related genes/proteins, lipids and common seen animal/experimental models with organized paragraphs from literatures.
What diseases are associated with FLAVONE?
FLAVONE is suspected in Diabetes Mellitus, Non-Insulin-Dependent, Obesity, Chronic Disease, Disintegration, Cardiovascular Diseases, Cerebrovascular accident and other diseases in descending order of the highest number of associated sentences.
Related references are mostly published in these journals:
| Disease | Cross reference | Weighted score | Related literature |
|---|
Possible diseases from mapped MeSH terms on references
We collected disease MeSH terms mapped to the references associated with FLAVONE
PubChem Associated disorders and diseases
What pathways are associated with FLAVONE
There are no associated biomedical information in the current reference collection.
PubChem Biomolecular Interactions and Pathways
Link to PubChem Biomolecular Interactions and PathwaysWhat cellular locations are associated with FLAVONE?
Visualization in cellular structure
Associated locations are in red color. Not associated locations are in black.
Related references are published most in these journals:
| Location | Cross reference | Weighted score | Related literatures |
|---|
What functions are associated with FLAVONE?
Related references are published most in these journals:
| Function | Cross reference | Weighted score | Related literatures |
|---|
What lipids are associated with FLAVONE?
Related references are published most in these journals:
| Lipid concept | Cross reference | Weighted score | Related literatures |
|---|
What genes are associated with FLAVONE?
Related references are published most in these journals:
| Gene | Cross reference | Weighted score | Related literatures |
|---|
What common seen animal models are associated with FLAVONE?
Knock-out
Knock-out are used in the study 'MATE2 mediates vacuolar sequestration of flavonoid glycosides and glycoside malonates in Medicago truncatula.' (Zhao J et al., 2011) and Knock-out are used in the study 'How can research on plants contribute to promoting human health?' (Martin C et al., 2011).
Disease model
Disease model are used in the study 'How can research on plants contribute to promoting human health?' (Martin C et al., 2011).
Animal Disease Models
Animal Disease Models are used in the study 'How can research on plants contribute to promoting human health?' (Martin C et al., 2011).
Related references are published most in these journals:
| Model | Cross reference | Weighted score | Related literatures |
|---|
NCBI Entrez Crosslinks
All references with FLAVONE
Download all related citations| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Vijayaraghavan R et al. | Comparative evaluation of some flavonoids and tocopherol acetate against the systemic toxicity induced by sulphur mustard. | 2008 | Indian J Pharmacol | pmid:20040938 |
| Matsuda H et al. | Inhibitory effects of thunberginols A and B isolated from Hydrangeae Dulcis Folium on mRNA expression of cytokines and on activation of activator protein-1 in RBL-2H3 cells. | 2008 | Phytomedicine | pmid:17950587 |
| Nilsson J et al. | Azaflavones compared to flavones as ligands to the benzodiazepine binding site of brain GABA(A) receptors. | 2008 | Bioorg. Med. Chem. Lett. | pmid:18851913 |
| Furtmueller R et al. | 6,3'-Dinitroflavone is a low efficacy modulator of GABA(A) receptors. | 2008 | Eur. J. Pharmacol. | pmid:18639544 |
| Yang L et al. | Compound Chinese herbal medicinal ingredients can enhance immune response and efficacy of RHD vaccine in rabbit. | 2008 | Vaccine | pmid:18602959 |
| Deng X et al. | Qualitative and quantitative analysis of flavonoids in the leaves of Isatis indigatica Fort. by ultra-performance liquid chromatography with PDA and electrospray ionization tandem mass spectrometry detection. | 2008 | J Pharm Biomed Anal | pmid:18585883 |
| Kong CH et al. | Fate and impact on microorganisms of rice allelochemicals in paddy soil. | 2008 | J. Agric. Food Chem. | pmid:18540621 |
| Nagar S et al. | Pharmacophore mapping of flavone derivatives for aromatase inhibition. | 2008 | Mol. Divers. | pmid:18506592 |
| Galajda Z et al. | [Cardiovascular actions of a standardized polyphenol concentrate on patients undergoing coronary bypass grafting: a randomized, double-blind, placebo-controlled study]. | 2008 | Magy Seb | pmid:18504236 |
| Mori M et al. | Cyanosalvianin, a supramolecular blue metalloanthocyanin, from petals of Salvia uliginosa. | 2008 | Phytochemistry | pmid:18466933 |
| Li WW and Zhang ZT | Hexaquacobalt(II) bis(5-hydroxy-7-methoxy-4-oxo-2-phenyl-4H-chromene-6-sulfonate) tetrahydrate. | 2008 | Acta Crystallogr C | pmid:18391382 |
| Mursu J et al. | Flavonoid intake and the risk of ischaemic stroke and CVD mortality in middle-aged Finnish men: the Kuopio Ischaemic Heart Disease Risk Factor Study. | 2008 | Br. J. Nutr. | pmid:18377681 |
| Winkelmann I et al. | Proteome response in HT-29 human colorectal cancer cells to two apoptosis-inducing compounds with different mode of action. | 2008 | Int. J. Cancer | pmid:18214853 |
| Yamada P et al. | Inhibitory effect of various Tunisian olive oils on chemical mediator release and cytokine production by basophilic cells. | 2008 | J Ethnopharmacol | pmid:18178046 |
| Djeridane A et al. | Inhibition of porcine liver carboxylesterase by a new flavone glucoside isolated from Deverra scoparia. | 2008 | Chem. Biol. Interact. | pmid:18163984 |
| Jandera P et al. | Optimization of separation in two-dimensional high-performance liquid chromatography by adjusting phase system selectivity and using programmed elution techniques. | 2008 | J Chromatogr A | pmid:18067903 |
| Seelinger G et al. | Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. | 2008 | Planta Med. | pmid:18937165 |
| Diniz A et al. | Characterization of interactions between polyphenolic compounds and human serum proteins by capillary electrophoresis. | 2008 | Anal Bioanal Chem | pmid:18418586 |
| Billard C et al. | Flavopiridol-induced iNOS downregulation during apoptosis of chronic lymphocytic leukemia cells is caspase-dependent. | 2008 | Leuk. Res. | pmid:17981326 |
| Chen HQ et al. | Luteolin protects dopaminergic neurons from inflammation-induced injury through inhibition of microglial activation. | 2008 | Neurosci. Lett. | pmid:18952146 |
| Nitoda T et al. | Effects of phenolic compounds isolated from Rabdosia japonica on B16-F10 melanoma cells. | 2008 | Phytother Res | pmid:18567053 |
| He XZ et al. | Regioselective synthesis of plant (iso)flavone glycosides in Escherichia coli. | 2008 | Appl. Microbiol. Biotechnol. | pmid:18568307 |
| Jiang L et al. | Analysis of flavonoids in propolis and Ginkgo biloba by micellar electrokinetic capillary chromatography. | 2008 | J. Agric. Food Chem. | pmid:19053353 |
| Meng Y et al. | Flavones and flavone glycosides from Halophila johnsonii. | 2008 | Phytochemistry | pmid:18771781 |
| Toulmin A et al. | Toward prediction of alkane/water partition coefficients. | 2008 | J. Med. Chem. | pmid:18558667 |
| March RE et al. | Empirically predicted 13C NMR chemical shifts for 8-hydroxyflavone starting from 7,8,4'-trihydroxyflavone and from 7,8-dihydroxyflavone. | 2008 | Magn Reson Chem | pmid:18389493 |
| Zhai K et al. | Chrysin induces hyperalgesia via the GABAA receptor in mice. | 2008 | Planta Med. | pmid:18612941 |
| Kumar S et al. | Pharmacological evaluation of Bioactive Principle of Turnera aphrodisiaca. | 2008 | Indian J Pharm Sci | pmid:21369434 |
| Ko JH et al. | Four glucosyltransferases from rice: cDNA cloning, expression, and characterization. | 2008 | J. Plant Physiol. | pmid:17363107 |
| Kaur P et al. | Plant flavonoid apigenin inactivates Akt to trigger apoptosis in human prostate cancer: an in vitro and in vivo study. | 2008 | Carcinogenesis | pmid:18725386 |
| Luo P et al. | Scutellarin isolated from Erigeron multiradiatus inhibits high glucose-mediated vascular inflammation. | 2008 | Yakugaku Zasshi | pmid:18758143 |
| Rezai-Zadeh K et al. | Apigenin and luteolin modulate microglial activation via inhibition of STAT1-induced CD40 expression. | 2008 | J Neuroinflammation | pmid:18817573 |
| Somerset SM and Johannot L | Dietary flavonoid sources in Australian adults. | 2008 | Nutr Cancer | pmid:18584477 |
| Wu B et al. | Metabonomic study on ageing: NMR-based investigation into rat urinary metabolites and the effect of the total flavone of Epimedium. | 2008 | Mol Biosyst | pmid:18633487 |
| Anbazhagan V et al. | Investigations on the fluorescence quenching of 2,3-diazabicyclo[2.2.2]oct-2-ene by certain flavonoids. | 2008 | J. Photochem. Photobiol. B, Biol. | pmid:18440819 |
| Barbera M et al. | The ability of coumarin-, flavanon- and flavonol-analogues of flavone acetic acid to stimulate human monocytes. | 2008 | Oncol. Rep. | pmid:18097594 |
| Ryu YB et al. | Inhibitory effects on mushroom tyrosinase by flavones from the stem barks of Morus lhou (S.) Koidz. | 2008 | J Enzyme Inhib Med Chem | pmid:18608767 |
| Russo EB | Cannabinoids in the management of difficult to treat pain. | 2008 | Ther Clin Risk Manag | pmid:18728714 |
| Lv W et al. | Jaceosidin induces apoptosis in human ovary cancer cells through mitochondrial pathway. | 2008 | J. Biomed. Biotechnol. | pmid:18769496 |
| Horvath DP et al. | Transcriptome analysis identifies novel responses and potential regulatory genes involved in seasonal dormancy transitions of leafy spurge (Euphorbia esula L.). | 2008 | BMC Genomics | pmid:19014493 |
| Rachchh MA and Jain SM | Gastroprotective effect of Benincasa hispida fruit extract. | 2008 | Indian J Pharmacol | pmid:21279184 |
| Wang YZ et al. | In vivo effects of Pain Relieving Plaster on closed soft tissue injury in rabbit ears. | 2008 | BMC Complement Altern Med | pmid:18793392 |
| Lawrence CJ et al. | MaizeGDB: The maize model organism database for basic, translational, and applied research. | 2008 | Int J Plant Genomics | pmid:18769488 |
| Agbottah E et al. | Two specific drugs, BMS-345541 and purvalanol A induce apoptosis of HTLV-1 infected cells through inhibition of the NF-kappaB and cell cycle pathways. | 2008 | AIDS Res Ther | pmid:18544167 |
| Liu B and Yang BL | Poly[(μ-5,7-dihydr-oxy-4-oxo-2-phenyl-4H-chromene-8-sulfonato)potassium(I)]. | 2008 | Acta Crystallogr Sect E Struct Rep Online | pmid:21581534 |
| Liu B and Yang BL | Hexaaqua-(5,7-dihydr-oxy-4-oxo-2-phenyl-4H-chromene-8-sulfonato)calcium(II) 5,7-dihydr-oxy-4-oxo-2-phenyl-4H-chromene-8-sulfonate trihydrate. | 2008 | Acta Crystallogr Sect E Struct Rep Online | pmid:21581174 |
| Ramachandran G et al. | 2-(5,7-Dimeth-oxy-4-oxo-4H-chromen-2-yl)phenyl 4-toluene-sulfonate. | 2008 | Acta Crystallogr Sect E Struct Rep Online | pmid:21203275 |
| Wang XL et al. | Effects of eleven flavonoids from the osteoprotective fraction of Drynaria fortunei (KUNZE) J. SM. on osteoblastic proliferation using an osteoblast-like cell line. | 2008 | Chem. Pharm. Bull. | pmid:18175973 |
| Yin J et al. | Antioxidant and antidiabetic activities of extracts from Cirsium japonicum roots. | 2008 | Nutr Res Pract | pmid:20016726 |
| Szliszka E et al. | Dietary flavonoids sensitize HeLa cells to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). | 2008 | Int J Mol Sci | pmid:19325719 |