| MeSH term | MeSH ID | Detail |
|---|---|---|
| Hemolysis | D006461 | 131 associated lipids |
| Stomach Ulcer | D013276 | 75 associated lipids |
| Diabetes Mellitus | D003920 | 90 associated lipids |
| Neoplasms, Experimental | D009374 | 10 associated lipids |
| Adenocarcinoma | D000230 | 166 associated lipids |
| Dermatitis, Contact | D003877 | 59 associated lipids |
| Pain | D010146 | 64 associated lipids |
| Lupus Erythematosus, Systemic | D008180 | 43 associated lipids |
| Lung Neoplasms | D008175 | 171 associated lipids |
| Pancreatic Neoplasms | D010190 | 77 associated lipids |
| Colonic Neoplasms | D003110 | 161 associated lipids |
| Diabetes Mellitus, Experimental | D003921 | 85 associated lipids |
| Mammary Neoplasms, Experimental | D008325 | 67 associated lipids |
| Body Weight | D001835 | 333 associated lipids |
| Edema | D004487 | 152 associated lipids |
| Prostatic Neoplasms | D011471 | 126 associated lipids |
| Osteosarcoma | D012516 | 50 associated lipids |
| Melanoma | D008545 | 69 associated lipids |
| Asthma | D001249 | 52 associated lipids |
| Glioma | D005910 | 112 associated lipids |
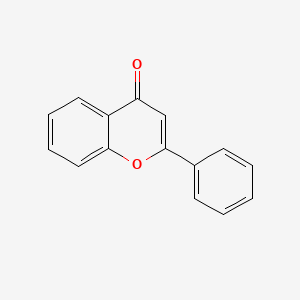
FLAVONE
FLAVONE is a lipid of Polyketides (PK) class. Flavone is associated with abnormalities such as Cardiovascular Diseases, Cerebrovascular accident, DERMATITIS HERPETIFORMIS, FAMILIAL, Hyperinsulinism and Inflammatory disorder. The involved functions are known as Oxidation-Reduction, Metabolic Inhibition, Inflammation, Phosphorylation and antioxidant activity. Flavone often locates in Endothelium, Hepatic, Protoplasm, Body tissue and Extracellular. The associated genes with FLAVONE are ICAM1 gene, BCL2L1 gene, MYC gene, TP53 gene and cytochrome c''. The related lipids are Promega, Steroids and Total cholesterol. The related experimental models are Knock-out, Disease model and Animal Disease Models.
Cross Reference
Introduction
To understand associated biological information of FLAVONE, we collected biological information of abnormalities, associated pathways, cellular/molecular locations, biological functions, related genes/proteins, lipids and common seen animal/experimental models with organized paragraphs from literatures.
What diseases are associated with FLAVONE?
FLAVONE is suspected in Diabetes Mellitus, Non-Insulin-Dependent, Obesity, Chronic Disease, Disintegration, Cardiovascular Diseases, Cerebrovascular accident and other diseases in descending order of the highest number of associated sentences.
Related references are mostly published in these journals:
| Disease | Cross reference | Weighted score | Related literature |
|---|
Possible diseases from mapped MeSH terms on references
We collected disease MeSH terms mapped to the references associated with FLAVONE
PubChem Associated disorders and diseases
What pathways are associated with FLAVONE
There are no associated biomedical information in the current reference collection.
PubChem Biomolecular Interactions and Pathways
Link to PubChem Biomolecular Interactions and PathwaysWhat cellular locations are associated with FLAVONE?
Visualization in cellular structure
Associated locations are in red color. Not associated locations are in black.
Related references are published most in these journals:
| Location | Cross reference | Weighted score | Related literatures |
|---|
What functions are associated with FLAVONE?
Related references are published most in these journals:
| Function | Cross reference | Weighted score | Related literatures |
|---|
What lipids are associated with FLAVONE?
Related references are published most in these journals:
| Lipid concept | Cross reference | Weighted score | Related literatures |
|---|
What genes are associated with FLAVONE?
Related references are published most in these journals:
| Gene | Cross reference | Weighted score | Related literatures |
|---|
What common seen animal models are associated with FLAVONE?
Knock-out
Knock-out are used in the study 'MATE2 mediates vacuolar sequestration of flavonoid glycosides and glycoside malonates in Medicago truncatula.' (Zhao J et al., 2011) and Knock-out are used in the study 'How can research on plants contribute to promoting human health?' (Martin C et al., 2011).
Disease model
Disease model are used in the study 'How can research on plants contribute to promoting human health?' (Martin C et al., 2011).
Animal Disease Models
Animal Disease Models are used in the study 'How can research on plants contribute to promoting human health?' (Martin C et al., 2011).
Related references are published most in these journals:
| Model | Cross reference | Weighted score | Related literatures |
|---|
NCBI Entrez Crosslinks
All references with FLAVONE
Download all related citations| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Yang L et al. | Compound Chinese herbal medicinal ingredients can enhance immune response and efficacy of RHD vaccine in rabbit. | 2008 | Vaccine | pmid:18602959 |
| Schrader KK | Plant Natural compounds with antibacterial activity towards common pathogens of pond-cultured channel catfish (Ictalurus punctatus). | 2010 | Toxins (Basel) | pmid:22069655 |
| Canivenc-Lavier MC et al. | Comparative effects of flavonoids and model inducers on drug-metabolizing enzymes in rat liver. | 1996 | Toxicology | pmid:8931757 |
| Ramful D et al. | Bioactive phenolics and antioxidant propensity of flavedo extracts of Mauritian citrus fruits: potential prophylactic ingredients for functional foods application. | 2010 | Toxicology | pmid:20100535 |
| Ebert B et al. | Phytochemicals induce breast cancer resistance protein in Caco-2 cells and enhance the transport of benzo[a]pyrene-3-sulfate. | 2007 | Toxicol. Sci. | pmid:17077187 |
| Sanderson JT et al. | Induction and inhibition of aromatase (CYP19) activity by natural and synthetic flavonoid compounds in H295R human adrenocortical carcinoma cells. | 2004 | Toxicol. Sci. | pmid:15319488 |
| Bugel SM et al. | Comparative Developmental Toxicity of Flavonoids Using an Integrative Zebrafish System. | 2016 | Toxicol. Sci. | pmid:27492224 |
| van Duursen MB et al. | Phytochemicals inhibit catechol-O-methyltransferase activity in cytosolic fractions from healthy human mammary tissues: implications for catechol estrogen-induced DNA damage. | 2004 | Toxicol. Sci. | pmid:15254334 |
| Hong H et al. | Human sex hormone-binding globulin binding affinities of 125 structurally diverse chemicals and comparison with their binding to androgen receptor, estrogen receptor, and α-fetoprotein. | 2015 | Toxicol. Sci. | pmid:25349334 |
| Ebert B et al. | Induction of phase-1 metabolizing enzymes by oltipraz, flavone and indole-3-carbinol enhance the formation and transport of benzo[a]pyrene sulfate conjugates in intestinal Caco-2 cells. | 2005 | Toxicol. Lett. | pmid:15890477 |
| Lim H et al. | Flavonoids interfere with NLRP3 inflammasome activation. | 2018 | Toxicol. Appl. Pharmacol. | pmid:29960001 |
| Khan R et al. | Chrysin protects against cisplatin-induced colon. toxicity via amelioration of oxidative stress and apoptosis: probable role of p38MAPK and p53. | 2012 | Toxicol. Appl. Pharmacol. | pmid:22155348 |
| Schutte ME et al. | An in vitro and in silico study on the flavonoid-mediated modulation of the transport of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) through Caco-2 monolayers. | 2006 | Toxicol. Appl. Pharmacol. | pmid:16997339 |
| Lin TY et al. | Hispidulin inhibits the release of glutamate in rat cerebrocortical nerve terminals. | 2012 | Toxicol. Appl. Pharmacol. | pmid:22759588 |
| Kamata R et al. | Agonistic effects of diverse xenobiotics on the constitutive androstane receptor as detected in a recombinant yeast-cell assay. | 2018 | Toxicol In Vitro | pmid:28927721 |
| Russo EB | Cannabinoids in the management of difficult to treat pain. | 2008 | Ther Clin Risk Manag | pmid:18728714 |
| Meyer JD et al. | Quantitative trait loci for maysin synthesis in maize (Zea mays L.) lines selected for high silk maysin content. | 2007 | Theor. Appl. Genet. | pmid:17486311 |
| Baranowska I and Raróg D | Application of derivative spectrophotometry to determination of flavonoid mixtures. | 2001 | Talanta | pmid:18968363 |
| Liu Q et al. | Metabolism profile of scutellarin in urine following oral administration to rats by ultra performance liquid chromatography coupled to time-of-flight mass spectrometry. | 2009 | Talanta | pmid:19782195 |
| Toyo'oka T et al. | On-line screening methods for antioxidants scavenging superoxide anion radical and hydrogen peroxide by liquid chromatography with indirect chemiluminescence detection. | 2003 | Talanta | pmid:18969068 |
| Zhang J et al. | A flavone-based turn-on fluorescent probe for intracellular cysteine/homocysteine sensing with high selectivity. | 2016 | Talanta | pmid:26695232 |
| Karvela E et al. | Deployment of response surface methodology to optimise recovery of grape (Vitis vinifera) stem polyphenols. | 2009 | Talanta | pmid:19635365 |
| Collins BM et al. | The estrogenic and antiestrogenic activities of phytochemicals with the human estrogen receptor expressed in yeast. | 1997 | Steroids | pmid:9090797 |
| Marian CM | Spin-forbidden transitions in flavone. | 2009 | Spectrochim Acta A Mol Biomol Spectrosc | pmid:19264543 |
| Mitra A et al. | Studies on the interaction of a synthetic nitro-flavone derivative with DNA: A multi-spectroscopic and molecular docking approach. | 2018 | Spectrochim Acta A Mol Biomol Spectrosc | pmid:29885634 |
| Vrielynck L et al. | Self-modelling analysis applied to nanosecond transient absorption spectroscopy of flavone: an aid to elucidate and characterise reaction intermediates. | 2002 | Spectrochim Acta A Mol Biomol Spectrosc | pmid:12396046 |
| Xu S et al. | A novel flavone-based fluorescent probe for relay recognition of HSO3(-) and Al(3+). | 2015 | Spectrochim Acta A Mol Biomol Spectrosc | pmid:25965168 |
| Chen Q et al. | Measurement of total flavone content in snow lotus (Saussurea involucrate) using near infrared spectroscopy combined with interval PLS and genetic algorithm. | 2010 | Spectrochim Acta A Mol Biomol Spectrosc | pmid:20338806 |
| MartÃnez-Richa A and Joseph-Nathan P | Carbon-13 CP-MAS nuclear magnetic resonance studies of teas. | 2003 | Solid State Nucl Magn Reson | pmid:12763559 |
| Xiao Z et al. | [The inhibitory effect of total flavonoids of hippophae on the activation of NF-kappa B by stretching cultured cardiac myocytes]. | 2003 | Sichuan Da Xue Xue Bao Yi Xue Ban | pmid:12947714 |
| Gooda Sahib N et al. | Plants' metabolites as potential antiobesity agents. | 2012 | ScientificWorldJournal | pmid:22666121 |
| de-Faria FM et al. | Antioxidant action of mangrove polyphenols against gastric damage induced by absolute ethanol and ischemia-reperfusion in the rat. | 2012 | ScientificWorldJournal | pmid:22654592 |
| Kostrzewa-Susłow E and Janeczko T | Microbial transformations of 7-hydroxyflavanone. | 2012 | ScientificWorldJournal | pmid:22654578 |
| Lasker JM et al. | In vivo activation of zoxazolamine metabolism by flavone. | 1982 | Science | pmid:7089530 |
| Duchowicz PR et al. | QSAR analysis on Spodoptera litura antifeedant activities for flavone derivatives. | 2009 | Sci. Total Environ. | pmid:19846206 |
| Zhang C et al. | Common and unique cis-acting elements mediate xanthotoxin and flavone induction of the generalist P450 CYP321A1. | 2014 | Sci Rep | pmid:25262756 |
| Yan X et al. | 58-F, a flavanone from Ophiopogon japonicus, prevents hepatocyte death by decreasing lysosomal membrane permeability. | 2016 | Sci Rep | pmid:27306715 |
| Zhao E and Mu Q | Phytoestrogen biological actions on Mammalian reproductive system and cancer growth. | 2011 Jan-Mar | Sci Pharm | pmid:21617769 |
| El-Alfy TS et al. | A New Flavonoid C-Glycoside from Celtis australis L. and Celtis occidentalis L. Leaves and Potential Antioxidant and Cytotoxic Activities. | 2011 Oct-Dec | Sci Pharm | pmid:22145118 |
| Zhao Q et al. | A specialized flavone biosynthetic pathway has evolved in the medicinal plant, Scutellaria baicalensis. | 2016 | Sci Adv | pmid:27152350 |
| Blonska M et al. | Effect of flavone derivatives on interleukin-1beta (IL-1beta) mRNA expression and IL-1beta protein synthesis in stimulated RAW 264.7 macrophages. | 2003 | Scand. J. Immunol. | pmid:12588662 |
| Barrellier MT | [Lymphedema: is there a treatment?]. | 1992 Jan-Feb | Rev Med Interne | pmid:1410875 |
| Dorneanu V et al. | [Determination of flavone derivatives. I. Quantitative determination of condensation products of rutin with epsiolon-aminocaproic acid]. | 1974 Oct-Dec | Rev Med Chir Soc Med Nat Iasi | pmid:4453725 |
| Khan SI et al. | Potential utility of natural products as regulators of breast cancer-associated aromatase promoters. | 2011 | Reprod. Biol. Endocrinol. | pmid:21693041 |
| Wang YJ et al. | Computational analysis for hepatic safety signals of constituents present in botanical extracts widely used by women in the United States for treatment of menopausal symptoms. | 2011 | Regul. Toxicol. Pharmacol. | pmid:20920542 |
| Vitcheva V et al. | Hepatoprotective effects of saponarin, isolated from Gypsophila trichotoma Wend. on cocaine-induced oxidative stress in rats. | 2011 | Redox Rep. | pmid:21722413 |
| Justino GC et al. | Electrospray ionization tandem mass spectrometry fragmentation of protonated flavone and flavonol aglycones: a re-examination. | 2009 | Rapid Commun. Mass Spectrom. | pmid:19089862 |
| Pham MH et al. | Characterization of monohydroxylated derivatives of the anticancer agent flavone-8-acetic acid by liquid chromatography with on-line UV and mass spectrometry. | 2007 | Rapid Commun. Mass Spectrom. | pmid:17891752 |
| Burns DC et al. | A combined nuclear magnetic resonance and computational study of monohydroxyflavones applied to product ion mass spectra. | 2007 | Rapid Commun. Mass Spectrom. | pmid:17216597 |
| Cai H et al. | A straightforward means of coupling preparative high-performance liquid chromatography and mass spectrometry. | 2002 | Rapid Commun. Mass Spectrom. | pmid:11870892 |