| MeSH term | MeSH ID | Detail |
|---|---|---|
| Osteosarcoma | D012516 | 50 associated lipids |
| Acne Vulgaris | D000152 | 35 associated lipids |
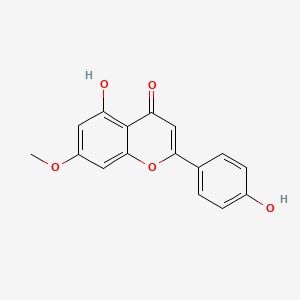
Genkwanin
Genkwanin is a lipid of Polyketides (PK) class. The involved functions are known as Synthesis, Gene Expression, nodulation, Signal Transduction and conjugation. The associated genes with Genkwanin are Genes, Bacterial.
Cross Reference
Introduction
To understand associated biological information of Genkwanin, we collected biological information of abnormalities, associated pathways, cellular/molecular locations, biological functions, related genes/proteins, lipids and common seen animal/experimental models with organized paragraphs from literatures.
What diseases are associated with Genkwanin?
There are no associated biomedical information in the current reference collection.
Possible diseases from mapped MeSH terms on references
We collected disease MeSH terms mapped to the references associated with Genkwanin
PubChem Associated disorders and diseases
What pathways are associated with Genkwanin
There are no associated biomedical information in the current reference collection.
PubChem Biomolecular Interactions and Pathways
Link to PubChem Biomolecular Interactions and PathwaysWhat cellular locations are associated with Genkwanin?
There are no associated biomedical information in the current reference collection.
What functions are associated with Genkwanin?
Related references are published most in these journals:
| Function | Cross reference | Weighted score | Related literatures |
|---|
What lipids are associated with Genkwanin?
There are no associated biomedical information in the current reference collection.
What genes are associated with Genkwanin?
Related references are published most in these journals:
| Gene | Cross reference | Weighted score | Related literatures |
|---|
What common seen animal models are associated with Genkwanin?
There are no associated biomedical information in the current reference collection.
NCBI Entrez Crosslinks
All references with Genkwanin
Download all related citations| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| pmid: | ||||
| Zhang J et al. | [Quality of processed samples of Daphne genkwa Sieb. et Zucc]. | 1997 | Zhongguo Zhong Yao Za Zhi | pmid:10743197 |
| Ahmed MS et al. | A weakly antimalarial biflavanone from Rhus retinorrhoea. | 2001 | Phytochemistry | pmid:11576606 |
| Zahid M et al. | Flavonoid glycosides from Salvia moorcroftiana wall. | 2002 | Carbohydr. Res. | pmid:11861014 |
| Yuan S et al. | [Comprehensive evaluation and practical confirmation on processing technology of Daphne genkwa Sieb. et Zucc]. | 1999 | Zhongguo Zhong Yao Za Zhi | pmid:12205864 |
| Ibañez E et al. | Subcritical water extraction of antioxidant compounds from rosemary plants. | 2003 | J. Agric. Food Chem. | pmid:12517098 |
| Kraft C et al. | In vitro antiplasmodial evaluation of medicinal plants from Zimbabwe. | 2003 | Phytother Res | pmid:12601673 |
| del Baño MJ et al. | Phenolic diterpenes, flavones, and rosmarinic acid distribution during the development of leaves, flowers, stems, and roots of Rosmarinus officinalis. Antioxidant activity. | 2003 | J. Agric. Food Chem. | pmid:12848492 |
| Santos-Gomes PC et al. | Determination of phenolic antioxidant compounds produced by calli and cell suspensions of sage (Salvia officinalis L.). | 2003 | J. Plant Physiol. | pmid:14593803 |
| Kim AR et al. | Active components from Artemisia iwayomogi displaying ONOO(-) scavenging activity. | 2004 | Phytother Res | pmid:14750192 |
| Cao S et al. | Cytotoxic triterpenoids from Acridocarpus vivy from the Madagascar rain forest. | 2004 | J. Nat. Prod. | pmid:15217279 |
| Martini ND et al. | Biological activity of five antibacterial flavonoids from Combretum erythrophyllum (Combretaceae). | 2004 | J Ethnopharmacol | pmid:15234754 |
| del Baño MJ et al. | Flavonoid distribution during the development of leaves, flowers, stems, and roots of Rosmarinus officinalis. postulation of a biosynthetic pathway. | 2004 | J. Agric. Food Chem. | pmid:15291464 |
| Zhang W et al. | [Studies on the chemical constituents in roots of Daphne odora var. atrocaulis]. | 2005 | Zhongguo Zhong Yao Za Zhi | pmid:16011095 |
| Leonard E et al. | Expression of a soluble flavone synthase allows the biosynthesis of phytoestrogen derivatives in Escherichia coli. | 2006 | Appl. Microbiol. Biotechnol. | pmid:16025328 |
| Park BY et al. | Isolation of flavonoids, a biscoumarin and an amide from the flower buds of Daphne genkwa and the evaluation of their anti-complement activity. | 2006 | Phytother Res | pmid:16685682 |
| Sadhu SK et al. | Prostaglandin inhibitory and antioxidant components of Cistus laurifolius, a Turkish medicinal plant. | 2006 | J Ethnopharmacol | pmid:16814498 |
| Pérez-Fons L et al. | Rosemary (Rosmarinus officinalis) diterpenes affect lipid polymorphism and fluidity in phospholipid membranes. | 2006 | Arch. Biochem. Biophys. | pmid:16949545 |
| Zhang YH et al. | [Studies on chemical constituents in spikes of Schizonepeta tenuifolia]. | 2006 | Zhongguo Zhong Yao Za Zhi | pmid:17048568 |
| Jones WP et al. | Cytotoxic constituents from the fruiting branches of Callicarpa americana collected in southern Florida. | 2007 | J. Nat. Prod. | pmid:17279798 |
| Hara H et al. | Laxative effect of agarwood leaves and its mechanism. | 2008 | Biosci. Biotechnol. Biochem. | pmid:18256503 |
| Saito T et al. | Sakuranetin induces adipogenesis of 3T3-L1 cells through enhanced expression of PPARgamma2. | 2008 | Biochem. Biophys. Res. Commun. | pmid:18522800 |
| Brozic P et al. | Flavonoids and cinnamic acid derivatives as inhibitors of 17beta-hydroxysteroid dehydrogenase type 1. | 2009 | Mol. Cell. Endocrinol. | pmid:18835421 |
| Su J et al. | Flavonoids from Daphne giraldii Nitsche. | 2008 | Nat. Prod. Res. | pmid:19023794 |
| Jeon YM et al. | Biological synthesis of 7-O-methyl Apigenin from naringenin using escherichia coli expressing two genes. | 2009 | J. Microbiol. Biotechnol. | pmid:19494697 |
| Androutsopoulos VP et al. | CYP1-mediated antiproliferative activity of dietary flavonoids in MDA-MB-468 breast cancer cells. | 2009 | Toxicology | pmid:19666078 |
| Henchiri H et al. | Sesquiterpenoids from Teucrium ramosissimum. | Phytochemistry | pmid:19766274 | |
| Pérez-Fons L et al. | Relationship between the antioxidant capacity and effect of rosemary (Rosmarinus officinalis L.) polyphenols on membrane phospholipid order. | 2010 | J. Agric. Food Chem. | pmid:19924866 |
| Tamaki Y et al. | Activated glutathione metabolism participates in protective effects of carnosic acid against oxidative stress in neuronal HT22 cells. | 2010 | Planta Med. | pmid:19941258 |
| Bai N et al. | Flavonoids and phenolic compounds from Rosmarinus officinalis. | 2010 | J. Agric. Food Chem. | pmid:20397728 |
| Mohammadi M et al. | Two new coumarins from the chloroform extract of Angelica urumiensis from Iran. | 2010 | Chem. Pharm. Bull. | pmid:20410639 |
| Lai HY et al. | Blechnum orientale Linn - a fern with potential as antioxidant, anticancer and antibacterial agent. | 2010 | BMC Complement Altern Med | pmid:20429956 |
| Liu RH et al. | [Studies on the chemical constituents from Daphne tangutica]. | 2009 | Zhong Yao Cai | pmid:20432900 |
| Devkota HP et al. | Flavonoids from the aerial parts of Diplomorpha canescens. | 2010 | Chem. Pharm. Bull. | pmid:20523001 |
| Brechenmacher L et al. | Soybean metabolites regulated in root hairs in response to the symbiotic bacterium Bradyrhizobium japonicum. | 2010 | Plant Physiol. | pmid:20534735 |
| Li YN et al. | A simple and efficient protocol for large-scale preparation of three flavonoids from the flower of Daphne genkwa by combination of macroporous resin and counter-current chromatography. | 2010 | J Sep Sci | pmid:20535750 |
| Tohno H et al. | Evaluation of estrogen receptor Beta binding of pruni cortex and its constituents. | 2010 | Yakugaku Zasshi | pmid:20606380 |
| Jordán MJ et al. | Introduction of distillate rosemary leaves into the diet of the Murciano-Granadina goat: transfer of polyphenolic compounds to goats' milk and the plasma of suckling goat kids. | 2010 | J. Agric. Food Chem. | pmid:20608728 |
| Mizuochi K et al. | New iridoid diesters of glucopyranose from Linaria canadensis (L.) Dum. | 2011 | J Nat Med | pmid:20635154 |
| Kunwar RM et al. | Traditional herbal medicine in far-west Nepal: a pharmacological appraisal. | 2010 | J Ethnobiol Ethnomed | pmid:21144003 |
| Androutsopoulos VP et al. | Comparative CYP1A1 and CYP1B1 substrate and inhibitor profile of dietary flavonoids. | 2011 | Bioorg. Med. Chem. | pmid:21482471 |
| Maia GL et al. | Flavonoids from Praxelis clematidea R.M. King and Robinson modulate bacterial drug resistance. | 2011 | Molecules | pmid:21666549 |
| Borrás Linares I et al. | Comparison of different extraction procedures for the comprehensive characterization of bioactive phenolic compounds in Rosmarinus officinalis by reversed-phase high-performance liquid chromatography with diode array detection coupled to electrospray time-of-flight mass spectrometry. | 2011 | J Chromatogr A | pmid:21835416 |
| Ben Sghaier M et al. | Flavonoids and sesquiterpenes from Tecurium ramosissimum promote antiproliferation of human cancer cells and enhance antioxidant activity: a structure-activity relationship study. | 2011 | Environ. Toxicol. Pharmacol. | pmid:22004952 |
| Piao GC et al. | Cytotoxic fraction from Artemisia sacrorum Ledeb. against three human cancer cell lines and separation and identification of its compounds. | 2012 | Nat. Prod. Res. | pmid:22008023 |
| Sati SC et al. | Bioactive constituents and medicinal importance of genus Alnus. | 2011 | Pharmacogn Rev | pmid:22279375 |
| Hyldgaard M et al. | Essential oils in food preservation: mode of action, synergies, and interactions with food matrix components. | 2012 | Front Microbiol | pmid:22291693 |
| Favela-Hernández JM et al. | Antibacterial and antimycobacterial lignans and flavonoids from Larrea tridentata. | 2012 | Phytother Res | pmid:22422605 |
| Escandón-Rivera S et al. | α-glucosidase inhibitors from Brickellia cavanillesii. | 2012 | J. Nat. Prod. | pmid:22587572 |
| Chang CW et al. | Daphne Genkwa sieb. Et zucc. Water-soluble extracts act on enterovirus 71 by inhibiting viral entry. | 2012 | Viruses | pmid:22590685 |