| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Wise ML et al. | Monoterpene synthases from common sage (Salvia officinalis). cDNA isolation, characterization, and functional expression of (+)-sabinene synthase, 1,8-cineole synthase, and (+)-bornyl diphosphate synthase. | 1998 | J. Biol. Chem. | pmid:9614092 |
| LaFever RE and Croteau R | Hydride shifts in the biosynthesis of the p-menthane monoterpenes alpha-terpinene, gamma-terpinene, and beta-phellandrene. | 1993 | Arch. Biochem. Biophys. | pmid:8460944 |
| Croteau R et al. | Biosynthesis of monoterpenes: partial purification, characterization, and mechanism of action of 1,8-cineole synthase. | 1994 | Arch. Biochem. Biophys. | pmid:8117108 |
| Pyun HJ et al. | Stereochemistry of the proton elimination in the formation of (+)- and (-)-alpha-pinene by monoterpene cyclases from sage (Salvia officinalis). | 1994 | Arch. Biochem. Biophys. | pmid:8109979 |
| Wagschal KC et al. | Monoterpene biosynthesis: isotope effects associated with bicyclic olefin formation catalyzed by pinene synthases from sage (Salvia officinalis). | 1994 | Arch. Biochem. Biophys. | pmid:8109978 |
| Pichersky E et al. | Purification and characterization of S-linalool synthase, an enzyme involved in the production of floral scent in Clarkia breweri. | 1995 | Arch. Biochem. Biophys. | pmid:7864636 |
| Appelkvist EL et al. | Regulation of coenzyme Q biosynthesis. | 1994 | Mol. Aspects Med. | pmid:7752843 |
| Croteau R et al. | Biosynthesis of monoterpenes: preliminary characterization of i-endo-fenchol synthetase from fennel (Foeniculum vulgare) and evidence that no free intermediate is involved in the cyclization of geranyl pyrophosphate to the rearranged product. | 1980 | Arch. Biochem. Biophys. | pmid:7436421 |
| Croteau R et al. | Biosynthesis of monoterpenes: conversion of the acyclic precursors geranyl pyrophosphate and neryl pyrophosphate to the rearranged monoterpenes fenchol and fenchone by a soluble enzyme preparation from fennel (Foeniculum vulgare). | 1980 | Arch. Biochem. Biophys. | pmid:7436420 |
| Croteau R and Felton M | Conversion of [1-3H2,G-14C]geranyl pyrophosphate to cyclic monoterpenes without loss of tritium. | 1981 | Arch. Biochem. Biophys. | pmid:7247415 |
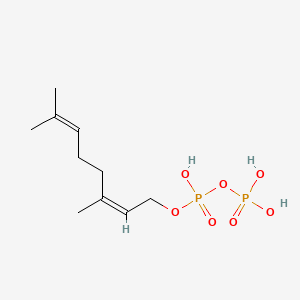
Neryl diphosphate
Neryl diphosphate is a lipid of Prenol Lipids (PR) class. The involved functions are known as Phenomenon. Neryl diphosphate often locates in Chloroplasts and Head. The associated genes with Neryl diphosphate are IPP gene.
Cross Reference
Introduction
To understand associated biological information of Neryl diphosphate, we collected biological information of abnormalities, associated pathways, cellular/molecular locations, biological functions, related genes/proteins, lipids and common seen animal/experimental models with organized paragraphs from literatures.
What diseases are associated with Neryl diphosphate?
There are no associated biomedical information in the current reference collection.
No disease MeSH terms mapped to the current reference collection.
PubChem Associated disorders and diseases
What pathways are associated with Neryl diphosphate
There are no associated biomedical information in the current reference collection.
PubChem Biomolecular Interactions and Pathways
Link to PubChem Biomolecular Interactions and PathwaysWhat cellular locations are associated with Neryl diphosphate?
Visualization in cellular structure
Associated locations are in red color. Not associated locations are in black.
Related references are published most in these journals:
| Location | Cross reference | Weighted score | Related literatures |
|---|
What functions are associated with Neryl diphosphate?
Related references are published most in these journals:
| Function | Cross reference | Weighted score | Related literatures |
|---|
What lipids are associated with Neryl diphosphate?
There are no associated biomedical information in the current reference collection.
What genes are associated with Neryl diphosphate?
Related references are published most in these journals:
| Gene | Cross reference | Weighted score | Related literatures |
|---|
What common seen animal models are associated with Neryl diphosphate?
There are no associated biomedical information in the current reference collection.