| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| pmid:28162767 | ||||
| pmid:14165506 | ||||
| pmid:28215610 | ||||
| pmid:27247193 | ||||
| pmid:27053081 | ||||
| pmid:27023458 | ||||
| pmid:24905149 | ||||
| pmid:24831707 | ||||
| pmid: | ||||
| Croteau R et al. | Biosynthesis of monoterpenes: conversion of the acyclic precursors geranyl pyrophosphate and neryl pyrophosphate to the rearranged monoterpenes fenchol and fenchone by a soluble enzyme preparation from fennel (Foeniculum vulgare). | 1980 | Arch. Biochem. Biophys. | pmid:7436420 |
| Croteau R et al. | Biosynthesis of monoterpenes: preliminary characterization of i-endo-fenchol synthetase from fennel (Foeniculum vulgare) and evidence that no free intermediate is involved in the cyclization of geranyl pyrophosphate to the rearranged product. | 1980 | Arch. Biochem. Biophys. | pmid:7436421 |
| Croteau R and Felton M | Conversion of [1-3H2,G-14C]geranyl pyrophosphate to cyclic monoterpenes without loss of tritium. | 1981 | Arch. Biochem. Biophys. | pmid:7247415 |
| Takahashi I and Ogura K | Farnesyl pyrophosphate synthetase from Bacillus subtilis. | 1981 | J. Biochem. | pmid:6792191 |
| Ness GC et al. | Influence of mevalonate kinase on studies of the MgATP-dependent inactivator of 3-hydroxy-3-methylglutaryl coenzyme A reductase. | 1982 | Arch. Biochem. Biophys. | pmid:6284035 |
| Gambliel H and Croteau R | Biosynthesis of (+/-)-alpha-pinene and (-)-beta-pinene from geranyl pyrophosphate by a soluble enzyme system from sage (Salvia officinalis). | 1982 | J. Biol. Chem. | pmid:7037765 |
| Cane DE | Cell-free studies of monoterpene and sesquiterpene biosynthesis. | 1983 | Biochem. Soc. Trans. | pmid:6642060 |
| Rojas MC et al. | Substrate and metal specificity in the enzymic synthesis of cyclic monoterpenes from geranyl and neryl pyrophosphate. | 1983 | Arch. Biochem. Biophys. | pmid:6847193 |
| Kjonaas R and Croteau R | Demonstration that limonene is the first cyclic intermediate in the biosynthesis of oxygenated p-menthane monoterpenes in Mentha piperita and other Mentha species. | 1983 | Arch. Biochem. Biophys. | pmid:6830247 |
| Gambliel H and Croteau R | Pinene cyclases I and II. Two enzymes from sage (Salvia officinalis) which catalyze stereospecific cyclizations of geranyl pyrophosphate to monoterpene olefins of opposite configuration. | 1984 | J. Biol. Chem. | pmid:6693393 |
| Croteau R et al. | Stereochemistry at C-1 of geranyl pyrophosphate and neryl pyrophosphate in the cyclization to (+)- and (-)-bornyl pyrophosphate. | 1985 | J. Biol. Chem. | pmid:3997807 |
| Sagami H and Ogura K | Geranylpyrophosphate synthetase-geranylgeranylpyrophosphate synthetase from Micrococcus luteus. | 1985 | Meth. Enzymol. | pmid:4021813 |
| Satterwhite DM et al. | Biosynthesis of monoterpenes. Enantioselectivity in the enzymatic cyclization of linalyl pyrophosphate to (-)-endo-fenchol. | 1985 | J. Biol. Chem. | pmid:4055764 |
| Croteau RB et al. | Mechanism of the pyrophosphate migration in the enzymatic cyclization of geranyl and linalyl pyrophosphates to (+)- and (-)-bornyl pyrophosphates. | 1985 | Biochemistry | pmid:4084562 |
| Cane DE | Stereochemical studies of natural products biosynthesis. | 1986 | Ann. N. Y. Acad. Sci. | pmid:3460491 |
| Fujisaki S et al. | Biosynthesis of isoprenoids in intact cells of Escherichia coli. | 1986 | J. Biochem. | pmid:3519600 |
| Wheeler CJ and Croteau RB | Direct demonstration of the isomerization component of the monoterpene cyclase reaction using a cyclopropylcarbinyl pyrophosphate substrate analog. | 1987 | Proc. Natl. Acad. Sci. U.S.A. | pmid:3474630 |
| Croteau R et al. | Biosynthesis of monoterpenes. Stereochemistry of the enzymatic cyclization of geranyl pyrophosphate to (-)-endo-fenchol. | 1988 | J. Biol. Chem. | pmid:3170591 |
| Hallahan TW and Croteau R | Monoterpene biosynthesis: demonstration of a geranyl pyrophosphate:sabinene hydrate cyclase in soluble enzyme preparations from sweet marjoram (Majorana hortensis). | 1988 | Arch. Biochem. Biophys. | pmid:3401015 |
| Croteau R and Purkett PT | Geranyl pyrophosphate synthase: characterization of the enzyme and evidence that this chain-length specific prenyltransferase is associated with monoterpene biosynthesis in sage (Salvia officinalis). | 1989 | Arch. Biochem. Biophys. | pmid:2730002 |
| Hallahan TW and Croteau R | Monoterpene biosynthesis: mechanism and stereochemistry of the enzymatic cyclization of geranyl pyrophosphate to (+)-cis- and (+)-trans-sabinene hydrate. | 1989 | Arch. Biochem. Biophys. | pmid:2916845 |
| Croteau R et al. | Biosynthesis of monoterpenes. Stereochemistry of the enzymatic cyclizations of geranyl pyrophosphate to (+)-alpha-pinene and (-)-beta-pinene. | 1989 | J. Biol. Chem. | pmid:2644252 |
| Light DR and Dennis MS | Purification of a prenyltransferase that elongates cis-polyisoprene rubber from the latex of Hevea brasiliensis. | 1989 | J. Biol. Chem. | pmid:2808388 |
| Light DR et al. | Rubber elongation by farnesyl pyrophosphate synthases involves a novel switch in enzyme stereospecificity. | 1989 | J. Biol. Chem. | pmid:2808389 |
| Wheeler CJ et al. | Uncompetitive inhibition of monoterpene cyclases by an analog of the substrate geranyl pyrophosphate and inhibition of monoterpene biosynthesis in vivo by an analog of geraniol. | 1990 | Arch. Biochem. Biophys. | pmid:2350172 |
| Croteau R et al. | Biosynthesis of monoterpenes: stereochemistry of the coupled isomerization and cyclization of geranyl pyrophosphate to camphane and isocamphane monoterpenes. | 1990 | Arch. Biochem. Biophys. | pmid:2178556 |
| Oulmouden A and Karst F | Nucleotide sequence of the ERG12 gene of Saccharomyces cerevisiae encoding mevalonate kinase. | 1991 | Curr. Genet. | pmid:1645230 |
| Lewinsohn E et al. | Wound-inducible pinene cyclase from grand fir: purification, characterization, and renaturation after SDS-PAGE. | 1992 | Arch. Biochem. Biophys. | pmid:1731633 |
| Ericsson J et al. | Substrate specificity of cis-prenyltransferase in rat liver microsomes. | 1992 | J. Biol. Chem. | pmid:1527094 |
| Alonso WR et al. | Purification of 4S-limonene synthase, a monoterpene cyclase from the glandular trichomes of peppermint (Mentha x piperita) and spearmint (Mentha spicata). | 1992 | J. Biol. Chem. | pmid:1559995 |
| McGeady P et al. | Biosynthesis of monoterpenes: inhibition of (+)-pinene and (-)-pinene cyclases by thia and aza analogs of the 4R- and 4S-alpha-terpinyl carbocation. | 1992 | Arch. Biochem. Biophys. | pmid:1444453 |
| LaFever RE and Croteau R | Hydride shifts in the biosynthesis of the p-menthane monoterpenes alpha-terpinene, gamma-terpinene, and beta-phellandrene. | 1993 | Arch. Biochem. Biophys. | pmid:8460944 |
| Croteau R et al. | Biosynthesis of monoterpenes: partial purification, characterization, and mechanism of action of 1,8-cineole synthase. | 1994 | Arch. Biochem. Biophys. | pmid:8117108 |
| Appelkvist EL et al. | Regulation of coenzyme Q biosynthesis. | 1994 | Mol. Aspects Med. | pmid:7752843 |
| Wagschal KC et al. | Monoterpene biosynthesis: isotope effects associated with bicyclic olefin formation catalyzed by pinene synthases from sage (Salvia officinalis). | 1994 | Arch. Biochem. Biophys. | pmid:8109978 |
| Pyun HJ et al. | Stereochemistry of the proton elimination in the formation of (+)- and (-)-alpha-pinene by monoterpene cyclases from sage (Salvia officinalis). | 1994 | Arch. Biochem. Biophys. | pmid:8109979 |
| Pichersky E et al. | Purification and characterization of S-linalool synthase, an enzyme involved in the production of floral scent in Clarkia breweri. | 1995 | Arch. Biochem. Biophys. | pmid:7864636 |
| Wise ML et al. | Monoterpene synthases from common sage (Salvia officinalis). cDNA isolation, characterization, and functional expression of (+)-sabinene synthase, 1,8-cineole synthase, and (+)-bornyl diphosphate synthase. | 1998 | J. Biol. Chem. | pmid:9614092 |
| Wagner PD and Vu ND | Phosphorylation of geranyl and farnesyl pyrophosphates by Nm23 proteins/nucleoside diphosphate kinases. | 2000 | J. Biol. Chem. | pmid:10952986 |
| Chang KC and Chuang NN | GTPase stimulation in shrimp Ras(Q(61)K) with geranylgeranyl pyrophosphate but not mammalian GAP. | 2001 | J. Exp. Zool. | pmid:11748613 |
| Montalvetti A et al. | Bisphosphonates are potent inhibitors of Trypanosoma cruzi farnesyl pyrophosphate synthase. | 2001 | J. Biol. Chem. | pmid:11435429 |
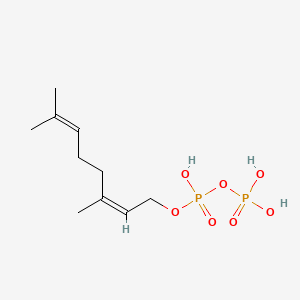
Neryl diphosphate
Neryl diphosphate is a lipid of Prenol Lipids (PR) class. The involved functions are known as Phenomenon. Neryl diphosphate often locates in Chloroplasts and Head. The associated genes with Neryl diphosphate are IPP gene.
Cross Reference
Introduction
To understand associated biological information of Neryl diphosphate, we collected biological information of abnormalities, associated pathways, cellular/molecular locations, biological functions, related genes/proteins, lipids and common seen animal/experimental models with organized paragraphs from literatures.
What diseases are associated with Neryl diphosphate?
There are no associated biomedical information in the current reference collection.
No disease MeSH terms mapped to the current reference collection.
PubChem Associated disorders and diseases
What pathways are associated with Neryl diphosphate
There are no associated biomedical information in the current reference collection.
PubChem Biomolecular Interactions and Pathways
Link to PubChem Biomolecular Interactions and PathwaysWhat cellular locations are associated with Neryl diphosphate?
Visualization in cellular structure
Associated locations are in red color. Not associated locations are in black.
Related references are published most in these journals:
| Location | Cross reference | Weighted score | Related literatures |
|---|
What functions are associated with Neryl diphosphate?
Related references are published most in these journals:
| Function | Cross reference | Weighted score | Related literatures |
|---|
What lipids are associated with Neryl diphosphate?
There are no associated biomedical information in the current reference collection.
What genes are associated with Neryl diphosphate?
Related references are published most in these journals:
| Gene | Cross reference | Weighted score | Related literatures |
|---|
What common seen animal models are associated with Neryl diphosphate?
There are no associated biomedical information in the current reference collection.