| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Moreno PR et al. | Induction of ajmalicine formation and related enzyme activities in Catharanthus roseus cells: effect of inoculum density. | 1993 | Appl. Microbiol. Biotechnol. | pmid:7763550 |
| Corsini A et al. | Relationship between mevalonate pathway and arterial myocyte proliferation: in vitro studies with inhibitors of HMG-CoA reductase. | 1993 | Atherosclerosis | pmid:8216498 |
| Blanchard L and Karst F | Characterization of a lysine-to-glutamic acid mutation in a conservative sequence of farnesyl diphosphate synthase from Saccharomyces cerevisiae. | 1993 | Gene | pmid:8096487 |
| Melnykovych G et al. | Growth inhibition of leukemia cell line CEM-C1 by farnesol: effects of phosphatidylcholine and diacylglycerol. | 1992 | Biochem. Biophys. Res. Commun. | pmid:1632790 |
| Moleyar V and Narasimham P | Antibacterial activity of essential oil components. | 1992 | Int. J. Food Microbiol. | pmid:1457292 |
| Baraldi PG et al. | Geiparvarin analogues. 3. Synthesis and cytostatic activity of 3(2H)-furanone and 4,5-dihydro-3(2H)-furanone congeners of geiparvarin, containing a geraniol-like fragment in the side chain. | 1992 | J. Med. Chem. | pmid:1588564 |
| Wang G et al. | [Analysis of chemical constituent of essential oil in Lonicera japonnica Thunb. cultivated on the northern plain of Henan Province]. | 1992 | Zhongguo Zhong Yao Za Zhi | pmid:1418559 |
| Van Dessel G et al. | Uptake of dolichol by Vero cells. | 1992 | Biochem. Cell Biol. | pmid:1449713 |
| Keung WM | Human liver alcohol dehydrogenases catalyze the oxidation of the intermediary alcohols of the shunt pathway of mevalonate metabolism. | 1991 | Biochem. Biophys. Res. Commun. | pmid:1993065 |
| Vilaplana J et al. | Contact dermatitis from geraniol in Bulgarian rose oil. | 1991 | Contact Derm. | pmid:1868720 |
| Chambon C et al. | Sterol pathway in yeast. Identification and properties of mutant strains defective in mevalonate diphosphate decarboxylase and farnesyl diphosphate synthetase. | 1991 | Lipids | pmid:1779710 |
| Shoff SM et al. | Concentration-dependent increase of murine P388 and B16 population doubling time by the acyclic monoterpene geraniol. | 1991 | Cancer Res. | pmid:1988098 |
| Wheeler CJ et al. | Uncompetitive inhibition of monoterpene cyclases by an analog of the substrate geranyl pyrophosphate and inhibition of monoterpene biosynthesis in vivo by an analog of geraniol. | 1990 | Arch. Biochem. Biophys. | pmid:2350172 |
| Chambon C et al. | Isolation and properties of yeast mutants affected in farnesyl diphosphate synthetase. | 1990 | Curr. Genet. | pmid:2245473 |
| Berger RG et al. | Catabolism of geraniol by cell suspension cultures of Citrus limon. | 1990 | Biochim. Biophys. Acta | pmid:2265211 |
| Hausen BM and Kulenkamp D | [Geraniol contact allergy]. | 1990 | Z. Hautkr. | pmid:2143044 |
| Matsuoka H et al. | Evaluation of antifungal volatile compounds on the basis of the elongation rate of a single hypha. | 1990 | Appl. Environ. Microbiol. | pmid:2082824 |
| Cardullo AC et al. | Allergic contact dermatitis resulting from sensitivity to citrus peel, geraniol, and citral. | 1989 | J. Am. Acad. Dermatol. | pmid:2526827 |
| Overbosch P et al. | Temporal integration and reaction times in human smell. | 1989 | Physiol. Behav. | pmid:2756054 |
| Kreilgård B and Hansen J | Aspects of pharmaceutical and chemical standardization of patch test materials. | 1989 | J. Am. Acad. Dermatol. | pmid:2600208 |
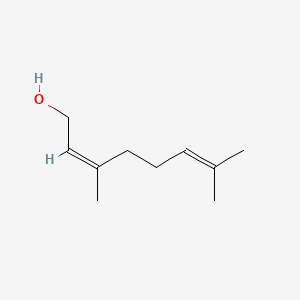
Nerol
Nerol is a lipid of Prenol Lipids (PR) class. The involved functions are known as Odorant, Anabolism, Diastasis, Metabolic Inhibition and Oxidation. Nerol often locates in germ tube. The related lipids are Octanols, Pinene, Hexanols, ethyl butyrate and ethyl hexanoate.
Cross Reference
Introduction
To understand associated biological information of Nerol, we collected biological information of abnormalities, associated pathways, cellular/molecular locations, biological functions, related genes/proteins, lipids and common seen animal/experimental models with organized paragraphs from literatures.
What diseases are associated with Nerol?
There are no associated biomedical information in the current reference collection.
No disease MeSH terms mapped to the current reference collection.
PubChem Associated disorders and diseases
What pathways are associated with Nerol
There are no associated biomedical information in the current reference collection.
PubChem Biomolecular Interactions and Pathways
Link to PubChem Biomolecular Interactions and PathwaysWhat cellular locations are associated with Nerol?
Visualization in cellular structure
Associated locations are in red color. Not associated locations are in black.
Related references are published most in these journals:
| Location | Cross reference | Weighted score | Related literatures |
|---|
What functions are associated with Nerol?
Related references are published most in these journals:
| Function | Cross reference | Weighted score | Related literatures |
|---|
What lipids are associated with Nerol?
Related references are published most in these journals:
| Lipid concept | Cross reference | Weighted score | Related literatures |
|---|
What genes are associated with Nerol?
There are no associated biomedical information in the current reference collection.
What common seen animal models are associated with Nerol?
There are no associated biomedical information in the current reference collection.