| MeSH term | MeSH ID | Detail |
|---|---|---|
| Adenocarcinoma | D000230 | 166 associated lipids |
| Lupus Erythematosus, Systemic | D008180 | 43 associated lipids |
| Lung Neoplasms | D008175 | 171 associated lipids |
| Pancreatic Neoplasms | D010190 | 77 associated lipids |
| Colonic Neoplasms | D003110 | 161 associated lipids |
| Mammary Neoplasms, Experimental | D008325 | 67 associated lipids |
| Body Weight | D001835 | 333 associated lipids |
| Edema | D004487 | 152 associated lipids |
| Precancerous Conditions | D011230 | 48 associated lipids |
| Prostatic Neoplasms | D011471 | 126 associated lipids |
| Liver Cirrhosis, Experimental | D008106 | 36 associated lipids |
| Osteosarcoma | D012516 | 50 associated lipids |
| Glioma | D005910 | 112 associated lipids |
| Cell Transformation, Neoplastic | D002471 | 126 associated lipids |
| Hypercholesterolemia | D006937 | 91 associated lipids |
| Thyroid Neoplasms | D013964 | 33 associated lipids |
| Arteriosclerosis | D001161 | 86 associated lipids |
| Neuroblastoma | D009447 | 66 associated lipids |
| Carcinoma, Hepatocellular | D006528 | 140 associated lipids |
| Fibrosis | D005355 | 23 associated lipids |
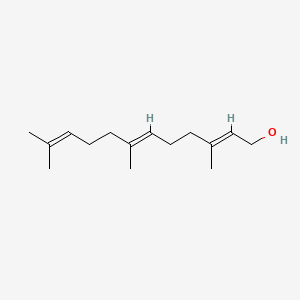
2E,6E-farnesol
2e,6e-farnesol is a lipid of Prenol Lipids (PR) class. 2e,6e-farnesol is associated with abnormalities such as Granulomatous Disease, Chronic, pathologic fistula and Cavitation. The involved functions are known as Regulation, Metabolic Inhibition, cholesterol biosynthetic process, Process and Transcription, Genetic. 2e,6e-farnesol often locates in Plasma membrane, Cytoplasmic matrix, cornified envelope, Epidermis and peroxisome. The associated genes with 2E,6E-farnesol are RAB3A gene, FOSL1 gene, CASP8AP2 gene, RCC1 gene and GALE gene. The related lipids are Sterols, Membrane Lipids and Steroids.
Cross Reference
Introduction
To understand associated biological information of 2E,6E-farnesol, we collected biological information of abnormalities, associated pathways, cellular/molecular locations, biological functions, related genes/proteins, lipids and common seen animal/experimental models with organized paragraphs from literatures.
What diseases are associated with 2E,6E-farnesol?
2E,6E-farnesol is suspected in Granulomatous Disease, Chronic, pathologic fistula and other diseases in descending order of the highest number of associated sentences.
Related references are mostly published in these journals:
| Disease | Cross reference | Weighted score | Related literature |
|---|
Possible diseases from mapped MeSH terms on references
We collected disease MeSH terms mapped to the references associated with 2E,6E-farnesol
PubChem Associated disorders and diseases
What pathways are associated with 2E,6E-farnesol
There are no associated biomedical information in the current reference collection.
PubChem Biomolecular Interactions and Pathways
Link to PubChem Biomolecular Interactions and PathwaysWhat cellular locations are associated with 2E,6E-farnesol?
Visualization in cellular structure
Associated locations are in red color. Not associated locations are in black.
Related references are published most in these journals:
| Location | Cross reference | Weighted score | Related literatures |
|---|
What functions are associated with 2E,6E-farnesol?
Related references are published most in these journals:
| Function | Cross reference | Weighted score | Related literatures |
|---|
What lipids are associated with 2E,6E-farnesol?
Related references are published most in these journals:
| Lipid concept | Cross reference | Weighted score | Related literatures |
|---|
What genes are associated with 2E,6E-farnesol?
Related references are published most in these journals:
| Gene | Cross reference | Weighted score | Related literatures |
|---|
What common seen animal models are associated with 2E,6E-farnesol?
There are no associated biomedical information in the current reference collection.
NCBI Entrez Crosslinks
All references with 2E,6E-farnesol
Download all related citations| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Rennemeier C et al. | Microbial quorum-sensing molecules induce acrosome loss and cell death in human spermatozoa. | 2009 | Infect. Immun. | pmid:19687207 |
| Sabra A et al. | Host-pathogen interaction and signaling molecule secretion are modified in the dpp3 knockout mutant of Candida lusitaniae. | 2014 | Infect. Immun. | pmid:24191303 |
| Navarathna DH et al. | Effect of farnesol on a mouse model of systemic candidiasis, determined by use of a DPP3 knockout mutant of Candida albicans. | 2007 | Infect. Immun. | pmid:17283095 |
| Navarathna DH et al. | Exogenous farnesol interferes with the normal progression of cytokine expression during candidiasis in a mouse model. | 2007 | Infect. Immun. | pmid:17517874 |
| Hargarten JC et al. | Candida albicans Quorum Sensing Molecules Stimulate Mouse Macrophage Migration. | 2015 | Infect. Immun. | pmid:26195556 |
| Mor A et al. | Inhibitory effect of farnesylthiosalicylic acid on mediators release by mast cells: preferential inhibition of prostaglandin D(2) and tumor necrosis factor-α release. | 2011 | Inflammation | pmid:20706780 |
| Mor A et al. | Celecoxib enhances the anti-inflammatory effects of farnesylthiosalicylic acid on T cells independent of prostaglandin E(2) production. | 2012 | Inflammation | pmid:22688643 |
| Rivera-Perez C et al. | Aldehyde dehydrogenase 3 converts farnesal into farnesoic acid in the corpora allata of mosquitoes. | 2013 | Insect Biochem. Mol. Biol. | pmid:23639754 |
| Sperry AE and Sen SE | Farnesol oxidation in insects: evidence that the biosynthesis of insect juvenile hormone is mediated by a specific alcohol oxidase. | 2001 | Insect Biochem. Mol. Biol. | pmid:11164339 |
| Sen SE et al. | Juvenile hormone biosynthesis in moths: synthesis and evaluation of farnesol homologs as alternate substrates of farnesol oxidase. | 2003 | Insect Biochem. Mol. Biol. | pmid:12770578 |
| Wozniak M et al. | Alternative farnesoid structures induce different conformational outcomes upon the Drosophila ortholog of the retinoid X receptor, ultraspiracle. | 2004 | Insect Biochem. Mol. Biol. | pmid:15522611 |
| Shintre MS et al. | Efficacy of an alcohol-based healthcare hand rub containing synergistic combination of farnesol and benzethonium chloride. | 2006 | Int J Hyg Environ Health | pmid:16750419 |
| Jeon JG et al. | Influences of trans-trans farnesol, a membrane-targeting sesquiterpenoid, on Streptococcus mutans physiology and survival within mixed-species oral biofilms. | 2011 | Int J Oral Sci | pmid:21485314 |
| Nokhodchi A et al. | The effect of terpene concentrations on the skin penetration of diclofenac sodium. | 2007 | Int J Pharm | pmid:17174049 |
| Winder BS and Roberts PE | Characterization of the proteins from Melanoplus bivittatus that bind juvenile hormone, and a refined EFDA photolabeling technique. | 1992 | Int. J. Biochem. | pmid:1426524 |
| Makovski V et al. | Farnesylthiosalicylic acid (salirasib) inhibits Rheb in TSC2-null ELT3 cells: a potential treatment for lymphangioleiomyomatosis. | 2012 | Int. J. Cancer | pmid:21500191 |
| Yaari-Stark S et al. | Ras inhibits endoplasmic reticulum stress in human cancer cells with amplified Myc. | 2010 | Int. J. Cancer | pmid:19998334 |
| Biran A et al. | Downregulation of survivin and aurora A by histone deacetylase and RAS inhibitors: a new drug combination for cancer therapy. | 2011 | Int. J. Cancer | pmid:20473860 |
| Egozi Y et al. | Growth inhibition of ras-dependent tumors in nude mice by a potent ras-dislodging antagonist. | 1999 | Int. J. Cancer | pmid:10074926 |
| Smalley KS and Eisen TG | Farnesyl thiosalicylic acid inhibits the growth of melanoma cells through a combination of cytostatic and pro-apoptotic effects. | 2002 | Int. J. Cancer | pmid:11920610 |
| Smalley KS and Eisen TG | Farnesyl transferase inhibitor SCH66336 is cytostatic, pro-apoptotic and enhances chemosensitivity to cisplatin in melanoma cells. | 2003 | Int. J. Cancer | pmid:12673674 |
| Yue W et al. | Farnesylthiosalicylic acid blocks mammalian target of rapamycin signaling in breast cancer cells. | 2005 | Int. J. Cancer | pmid:15957161 |
| Blum R et al. | E2F1 identified by promoter and biochemical analysis as a central target of glioblastoma cell-cycle arrest in response to Ras inhibition. | 2006 | Int. J. Cancer | pmid:16496386 |
| Beiner ME et al. | Ras antagonist inhibits growth and chemosensitizes human epithelial ovarian cancer cells. | 2006 Jan-Feb | Int. J. Gynecol. Cancer | pmid:16515591 |
| Davey KG and Sommerville RI | Molting in a parasitic nematode, Phocanema decipiens. VII. The mode of action of the ecdysial hormone. | 1974 | Int. J. Parasitol. | pmid:4853251 |
| Tofts J and Meerovitch E | The effect of farnesyl methyl ether, a mimic of insect juvenile hormone, on Hymenolepis Diminuta in vitro. | 1974 | Int. J. Parasitol. | pmid:4822487 |
| Davey KG | Molting in a parasitic nematode, Phocanema decipiens. VI. The mode of action of insect juvenile hormone and farnesyl methyl ether. | 1971 | Int. J. Parasitol. | pmid:5161204 |
| Laheru D et al. | Integrated preclinical and clinical development of S-trans, trans-Farnesylthiosalicylic Acid (FTS, Salirasib) in pancreatic cancer. | 2012 | Invest New Drugs | pmid:22547163 |
| Eglin D et al. | Farnesol-modified biodegradable polyurethanes for cartilage tissue engineering. | 2010 | J Biomed Mater Res A | pmid:19191318 |
| Kraitzer A et al. | Mechanisms of antiproliferative drug release from bioresorbable porous structures. | 2013 | J Biomed Mater Res A | pmid:23065767 |
| Unnanuntana A et al. | The effects of farnesol on Staphylococcus aureus biofilms and osteoblasts. An in vitro study. | 2009 | J Bone Joint Surg Am | pmid:19884443 |
| Kubesová A et al. | Separation of attogram terpenes by the capillary zone electrophoresis with fluorometric detection. | 2010 | J Chromatogr A | pmid:20933239 |
| Cagliero C et al. | Analysis of essential oils and fragrances with a new generation of highly inert gas chromatographic columns coated with ionic liquids. | 2017 | J Chromatogr A | pmid:28343686 |
| Lee H et al. | Characterization of (E,E)-farnesol and its fatty acid esters from anal scent glands of nutria (Myocastor coypus) by gas chromatography-mass spectrometry and gas chromatography-infrared spectrometry. | 2007 | J Chromatogr A | pmid:17709112 |
| Kang L et al. | SMGA gels for the skin permeation of haloperidol. | 2005 | J Control Release | pmid:15975680 |
| Novák VJ | Morphogenetic analysis of the effects of juvenile hormone analogues and other morphogenetically active substances on embryos of Schistocerca gregaria (Forskål). | 1969 | J Embryol Exp Morphol | pmid:5765790 |
| Woodard B et al. | 3D-QSAR of fungal quorum-sensing inhibiting analogs of farnesol. | 2007 Mar-Apr | J Environ Sci Health B | pmid:17454380 |
| Jahangir T and Sultana S | Benzo(a)pyrene-induced genotoxicity: attenuation by farnesol in a mouse model. | 2008 | J Enzyme Inhib Med Chem | pmid:18618320 |
| Rüegg T et al. | 3-Farnesyl-2-hydroxybenzoic acid is a new anti-Helicobacter pylori compound from Piper multiplinervium. | 2006 | J Ethnopharmacol | pmid:16266794 |
| Huang J et al. | Electrohydrodynamic deposition of nanotitanium doped hydroxyapatite coating for medical and dental applications. | 2011 | J Mater Sci Mater Med | pmid:21243517 |
| Jamalian A et al. | Chemical composition and antifungal activity of Matricaria recutita flower essential oil against medically important dermatophytes and soil-borne pathogens. | 2012 | J Mycol Med | pmid:23518164 |
| Gregus P et al. | Ultra high performance liquid chromatography tandem mass spectrometry analysis of quorum-sensing molecules of Candida albicans. | 2010 | J Pharm Biomed Anal | pmid:20580513 |
| Teshima K and Kondo T | Analytical method for determination of allylic isoprenols in rat tissues by liquid chromatography/tandem mass spectrometry following chemical derivatization with 3-nitrophtalic anhydride. | 2008 | J Pharm Biomed Anal | pmid:18313250 |
| Wang Z et al. | Farnesol for aerosol inhalation: nebulization and activity against human lung cancer cells. | 2003 Jan-Apr | J Pharm Pharm Sci | pmid:12753732 |
| Kraitzer A et al. | Composite fiber structures with antiproliferative agents exhibit advantageous drug delivery and cell growth inhibition in vitro. | 2011 | J Pharm Sci | pmid:20623695 |
| Abu-Mustafa EA et al. | Natural coumarins. XII. Umbelliprenin, a constituent of ammi majus L. fruits. | 1971 | J Pharm Sci | pmid:5125784 |
| Wartenberg D et al. | Proteome analysis of the farnesol-induced stress response in Aspergillus nidulans--The role of a putative dehydrin. | 2012 | J Proteomics | pmid:22634043 |
| Riely GJ et al. | A phase II trial of Salirasib in patients with lung adenocarcinomas with KRAS mutations. | 2011 | J Thorac Oncol | pmid:21847063 |
| Okazaki S et al. | [A 13-week subcutaneous toxicity study of prednisolone farnesylate (PNF) in rats]. | 1992 | J Toxicol Sci | pmid:1293320 |
| Nagashima Y et al. | [A 13-week percutaneous toxicity study of prednisolone farnesylate (PNF) gel in beagle dogs with a recovery period of 5 weeks]. | 1992 | J Toxicol Sci | pmid:1293321 |
| Nagashima Y et al. | [A 52-week percutaneous toxicity study of prednisolone farnesylate (PNF) gel in beagle dogs with a recovery period of 8 weeks]. | 1992 | J Toxicol Sci | pmid:1293322 |
| Taniguchi H et al. | [Reproductive and developmental toxicity study of prednisolone farnesylate (PNF)--study by subcutaneous administration of PNF prior to and in the early stages of pregnancy in rats]. | 1992 | J Toxicol Sci | pmid:1293323 |
| Taniguchi H et al. | [Reproductive and developmental toxicity study of prednisolone farnesylate (PNF)--study by subcutaneous administration of PNF during the period of fetal organogenesis in rats]. | 1992 | J Toxicol Sci | pmid:1293324 |
| Aso S et al. | [Reproductive and developmental toxicity study of prednisolone farnesylate (PNF)--teratogenicity study in rabbits by subcutaneous administration]. | 1992 | J Toxicol Sci | pmid:1293325 |
| Taniguchi H et al. | [Reproductive and developmental toxicity study of prednisolone farnesylate (PNF)--study of subcutaneous administration of PNF during the perinatal and lactation periods in rats]. | 1992 | J Toxicol Sci | pmid:1293326 |
| Otsuka M et al. | [Mutagenicity studies of prednisolone farnesylate (PNF)]. | 1992 | J Toxicol Sci | pmid:1293327 |
| Uchiyama H et al. | [Effects of prednisolone farnesylate (PNF) gel on skin and ocular mucosa]. | 1992 | J Toxicol Sci | pmid:1293328 |
| Okazaki S et al. | [A 13-week dermal toxicity study of prednisolone farnesylate (PNF) gel in rats with a recovery period of 5 weeks]. | 1992 | J Toxicol Sci | pmid:1293330 |
| Okazaki S et al. | [A 52-week dermal toxicity study of prednisolone farnesylate (PNF) gel in rats with a recovery period of 8 weeks]. | 1992 | J Toxicol Sci | pmid:1293331 |
| González-Coloma A et al. | Structure- and species-dependent insecticidal effects of neo-clerodane diterpenes. | 2000 | J. Agric. Food Chem. | pmid:10956169 |
| Ortiz de Montellano PR et al. | Letter: Squalene synthetase. Differentiation between the two substrate binding sites by a substrate analogue. | 1976 | J. Am. Chem. Soc. | pmid:1254859 |
| Tanaka S et al. | Stereoselective epoxidations of acyclic allylic alcohols by transition metal--hydroperoxide reagents. Synthesis of dl-C18 Cecropia juvenile hormone from farnesol. | 1974 | J. Am. Chem. Soc. | pmid:4854400 |
| van Tamelen EE and McCormick JP | Synthesis of cecropia juvenile hormone from trans,trans-farnesol. | 1970 | J. Am. Chem. Soc. | pmid:5411064 |
| Imai K et al. | Letter: Derivation of (plus)- and (minus)-C17-juvenile hormone from its racemic alcohol derivative via fungal metabolism. | 1974 | J. Am. Chem. Soc. | pmid:4414179 |
| Radetich B and Corey EJ | A general stereocontrolled, convergent synthesis of oligoprenols that parallels the biosynthetic pathway. | 2002 | J. Am. Chem. Soc. | pmid:11890779 |
| Clarke HC et al. | Ras antagonist farnesylthiosalicylic acid (FTS) reduces glomerular cellular proliferation and macrophage number in rat thy-1 nephritis. | 2003 | J. Am. Soc. Nephrol. | pmid:12660318 |
| Williams JM and Savage CO | Characterization of the regulation and functional consequences of p21ras activation in neutrophils by antineutrophil cytoplasm antibodies. | 2005 | J. Am. Soc. Nephrol. | pmid:15548565 |
| Nozawa Y et al. | Stachybotrin C and parvisporin, novel neuritogenic compounds. II. Structure determination. | 1997 | J. Antibiot. | pmid:9315075 |
| Cerca N et al. | Farnesol induces cell detachment from established S. epidermidis biofilms. | 2013 | J. Antibiot. | pmid:23549353 |
| Kaneko M et al. | Effect of farnesol on mevalonate pathway of Staphylococcus aureus. | 2011 | J. Antibiot. | pmid:21772307 |
| Kuroda M et al. | Sesquiterpene farnesol inhibits recycling of the C55 lipid carrier of the murein monomer precursor contributing to increased susceptibility to beta-lactams in methicillin-resistant Staphylococcus aureus. | 2007 | J. Antimicrob. Chemother. | pmid:17242033 |
| Koo H et al. | Inhibition of Streptococcus mutans biofilm accumulation and polysaccharide production by apigenin and tt-farnesol. | 2003 | J. Antimicrob. Chemother. | pmid:14563892 |
| Katragkou A et al. | In vitro interactions between farnesol and fluconazole, amphotericin B or micafungin against Candida albicans biofilms. | 2015 | J. Antimicrob. Chemother. | pmid:25288679 |
| Navarathna DH et al. | Enhanced pathogenicity of Candida albicans pre-treated with subinhibitory concentrations of fluconazole in a mouse model of disseminated candidiasis. | 2005 | J. Antimicrob. Chemother. | pmid:16239285 |
| Berrocal A et al. | Quorum sensing activity in Ophiostoma ulmi: effects of fusel oils and branched chain amino acids on yeast-mycelial dimorphism. | 2012 | J. Appl. Microbiol. | pmid:22519968 |
| Miyakawa T et al. | Role of metabolism of the mating pheromone in sexual differentiation of the heterobasidiomycete Rhodosporidium toruloides. | 1982 | J. Bacteriol. | pmid:7050081 |
| Machida K et al. | Farnesol-induced generation of reactive oxygen species via indirect inhibition of the mitochondrial electron transport chain in the yeast Saccharomyces cerevisiae. | 1998 | J. Bacteriol. | pmid:9721283 |
| Cantwell SG et al. | Biodegradation of acyclic isoprenoids by Pseudomonas species. | 1978 | J. Bacteriol. | pmid:681275 |
| Lorek J et al. | Influence of farnesol on the morphogenesis of Aspergillus niger. | 2008 | J. Basic Microbiol. | pmid:18383232 |
| Wendland J et al. | Use of the Porcine Intestinal Epithelium (PIE)-Assay to analyze early stages of colonization by the human fungal pathogen Candida albicans. | 2006 | J. Basic Microbiol. | pmid:17139615 |
| Saito Y and Ogura K | Biosynthesis of menaquinones. Enzymatic prenylation of 1,4-dihydroxy-2-naphthoate by Micrococcus luteus membrane fractions. | 1981 | J. Biochem. | pmid:7275947 |
| Nishino T et al. | Enzymatic formation of nerolidol in cell-free extract of Rhodotorula glutinis. | 1982 | J. Biochem. | pmid:6891702 |
| Takahashi I and Ogura K | Farnesyl pyrophosphate synthetase from Bacillus subtilis. | 1981 | J. Biochem. | pmid:6792191 |
| Hamada M et al. | Inhibitory activity of 1-farnesylpyridinium on the spatial control over the assembly of cell wall polysaccharides in Schizosaccharomyces pombe. | 2006 | J. Biochem. | pmid:17092950 |
| Ivanov SS et al. | Lipidation by the host prenyltransferase machinery facilitates membrane localization of Legionella pneumophila effector proteins. | 2010 | J. Biol. Chem. | pmid:20813839 |
| Gibbs JB et al. | Selective inhibition of farnesyl-protein transferase blocks ras processing in vivo. | 1993 | J. Biol. Chem. | pmid:8463291 |
| Kisselev O et al. | Efficient interaction with a receptor requires a specific type of prenyl group on the G protein gamma subunit. | 1995 | J. Biol. Chem. | pmid:7592699 |
| Fujiyama A et al. | S-farnesylation and methyl esterification of C-terminal domain of yeast RAS2 protein prior to fatty acid acylation. | 1991 | J. Biol. Chem. | pmid:1917931 |
| Maltese WA and Erdman RA | Characterization of isoprenoid involved in the post-translational modification of mammalian cell proteins. | 1989 | J. Biol. Chem. | pmid:2808372 |
| Gonzalez-Pacanowska D et al. | Isopentenoid synthesis in isolated embryonic Drosophila cells. Farnesol catabolism and omega-oxidation. | 1988 | J. Biol. Chem. | pmid:3335546 |
| Bostedor RG et al. | Farnesol-derived dicarboxylic acids in the urine of animals treated with zaragozic acid A or with farnesol. | 1997 | J. Biol. Chem. | pmid:9083051 |
| Koeppe JK et al. | A specific photoaffinity label for hemolymph and ovarian juvenile hormone-binding proteins in Leucophaea maderae. | 1984 | J. Biol. Chem. | pmid:6699014 |
| McGuire TF et al. | Platelet-derived growth factor receptor tyrosine phosphorylation requires protein geranylgeranylation but not farnesylation. | 1996 | J. Biol. Chem. | pmid:8910319 |
| Rosado JA and Sage SO | Role of the ERK pathway in the activation of store-mediated calcium entry in human platelets. | 2001 | J. Biol. Chem. | pmid:11278479 |
| Wright MM et al. | Uncoupling farnesol-induced apoptosis from its inhibition of phosphatidylcholine synthesis. | 2001 | J. Biol. Chem. | pmid:11306571 |
| Daily D et al. | Glutaredoxin protects cerebellar granule neurons from dopamine-induced apoptosis by dual activation of the ras-phosphoinositide 3-kinase and jun n-terminal kinase pathways. | 2001 | J. Biol. Chem. | pmid:11290748 |
| Correll CC et al. | Identification of farnesol as the non-sterol derivative of mevalonic acid required for the accelerated degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. | 1994 | J. Biol. Chem. | pmid:8021239 |
| Cox AD et al. | The CAAX peptidomimetic compound B581 specifically blocks farnesylated, but not geranylgeranylated or myristylated, oncogenic ras signaling and transformation. | 1994 | J. Biol. Chem. | pmid:8034681 |
| de Ropp JS and Troy FA | 2H NMR investigation of the organization and dynamics of polyisoprenols in membranes. | 1985 | J. Biol. Chem. | pmid:4066690 |
| Touhara K and Prestwich GD | Juvenile hormone epoxide hydrolase. Photoaffinity labeling, purification, and characterization from tobacco hornworm eggs. | 1993 | J. Biol. Chem. | pmid:8396141 |