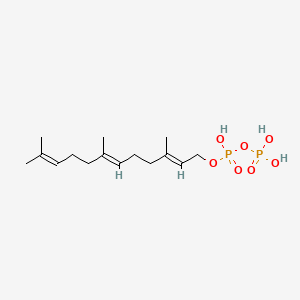
| Tachibana A et al. |
Evidence for farnesol-mediated isoprenoid synthesis regulation in a halophilic archaeon, Haloferax volcanii. |
1996 |
FEBS Lett. |
pmid:8566226
|
| Keller RK |
Squalene synthase inhibition alters metabolism of nonsterols in rat liver. |
1996 |
Biochim. Biophys. Acta |
pmid:8908150
|
| Krisans SK |
Cell compartmentalization of cholesterol biosynthesis. |
1996 |
Ann. N. Y. Acad. Sci. |
pmid:8993542
|
| Otto JC and Casey PJ |
The hepatitis delta virus large antigen is farnesylated both in vitro and in animal cells. |
1996 |
J. Biol. Chem. |
pmid:8617711
|
| Cane DE et al. |
Trichodiene synthase. Probing the role of the highly conserved aspartate-rich region by site-directed mutagenesis. |
1996 |
Biochemistry |
pmid:8823172
|
| Goalstone ML et al. |
Characterization of Xenopus laevis oocyte farnesyl transferase. |
1996 |
Biol. Reprod. |
pmid:8835391
|
| Williams TM et al. |
2-substituted piperazines as constrained amino acids. Application to the synthesis of potent, non carboxylic acid inhibitors of farnesyltransferase. |
1996 |
J. Med. Chem. |
pmid:8691462
|
| Parmryd I and Dallner G |
Organization of isoprenoid biosynthesis. |
1996 |
Biochem. Soc. Trans. |
pmid:8878825
|
| Scholten JD et al. |
Inhibitors of farnesyl:protein transferase--a possible cancer chemotherapeutic. |
1996 |
Bioorg. Med. Chem. |
pmid:8894110
|
| Dietrich A et al. |
Isoprenylation of the G protein gamma subunit is both necessary and sufficient for beta gamma dimer-mediated stimulation of phospholipase C. |
1996 |
Biochemistry |
pmid:8952464
|
| Lindsey S and Harwood HJ |
Inhibition of mammalian squalene synthetase activity by zaragozic acid A is a result of competitive inhibition followed by mechanism-based irreversible inactivation. |
1995 |
J. Biol. Chem. |
pmid:7721822
|
| Soltis DA et al. |
Expression, purification, and characterization of the human squalene synthase: use of yeast and baculoviral systems. |
1995 |
Arch. Biochem. Biophys. |
pmid:7864626
|
| Cane DE et al. |
Trichodiene synthase. Substrate specificity and inhibition. |
1995 |
Biochemistry |
pmid:7873526
|
| Cane DE et al. |
Trichodiene synthase. Identification of active site residues by site-directed mutagenesis. |
1995 |
Biochemistry |
pmid:7873527
|
| Harwood HJ |
Protein farnesyltransferase: measurement of enzymatic activity in 96-well format using TopCount microplate scintillation counting technology. |
1995 |
Anal. Biochem. |
pmid:7793628
|
| Cohen LH et al. |
Different analogues of farnesyl pyrophosphate inhibit squalene synthase and protein:farnesyltransferase to different extents. |
1995 |
Biochem. Pharmacol. |
pmid:7702642
|
| Furfine ES et al. |
Protein farnesyltransferase: kinetics of farnesyl pyrophosphate binding and product release. |
1995 |
Biochemistry |
pmid:7756316
|
| Dolence JM and Poulter CD |
A mechanism for posttranslational modifications of proteins by yeast protein farnesyltransferase. |
1995 |
Proc. Natl. Acad. Sci. U.S.A. |
pmid:7761439
|
| Cox AD |
Mutation and analysis of prenylation signal sequences. |
1995 |
Meth. Enzymol. |
pmid:7651143
|
| Thissen JA et al. |
Prenylated peptides in identification of specific binding proteins. |
1995 |
Meth. Enzymol. |
pmid:7651148
|
| Reiss Y |
Substrate interactions of protein prenyltransferases. |
1995 |
Meth. Enzymol. |
pmid:7651152
|
| Caplin BE and Marshall MS |
Mutagenesis and biochemical analysis of recombinant yeast prenyltransferases. |
1995 |
Meth. Enzymol. |
pmid:7651175
|
| Chen XY et al. |
Cloning, expression, and characterization of (+)-delta-cadinene synthase: a catalyst for cotton phytoalexin biosynthesis. |
1995 |
Arch. Biochem. Biophys. |
pmid:8554317
|
| Wiedłocha A et al. |
Translocation of cytosol of exogenous, CAAX-tagged acidic fibroblast growth factor. |
1995 |
J. Biol. Chem. |
pmid:8530506
|
| Khan SG et al. |
A rapid and convenient filter-binding assay for ras p21 processing enzyme farnesyltransferase. |
1995 |
J. Biochem. Biophys. Methods |
pmid:7494090
|
| Patel DV et al. |
Farnesyl diphosphate-based inhibitors of Ras farnesyl protein transferase. |
1995 |
J. Med. Chem. |
pmid:7636851
|
| McGeady P et al. |
The farnesyl group of H-Ras facilitates the activation of a soluble upstream activator of mitogen-activated protein kinase. |
1995 |
J. Biol. Chem. |
pmid:7592846
|
| Runquist M et al. |
Distribution of branch point prenyltransferases in regions of bovine brain. |
1995 |
J. Neurochem. |
pmid:7595519
|
| Tanaka Y et al. |
Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. |
1995 |
Nature |
pmid:7753173
|
| Vogt A et al. |
Burkitt lymphoma Daudi cells contain two distinct farnesyltransferases with different divalent cation requirements. |
1995 |
Biochemistry |
pmid:7547984
|
| Danesi R et al. |
Specific labeling of isoprenylated proteins: application to study inhibitors of the post-translational farnesylation and geranylgeranylation. |
1995 |
Biochem. Biophys. Res. Commun. |
pmid:7826382
|
| Epand RM et al. |
Lipid-mediated a-factor interactions with artificial membranes. |
1995 |
Meth. Enzymol. |
pmid:7651149
|
| Parmryd I et al. |
Identification of spinach farnesyl protein transferase. Dithiothreitol as an acceptor in vitro. |
1995 |
Eur. J. Biochem. |
pmid:8575428
|
| Thelin A et al. |
Effect of squalestatin 1 on the biosynthesis of the mevalonate pathway lipids. |
1994 |
Biochim. Biophys. Acta |
pmid:7811707
|
| Bansal VS and Vaidya S |
Characterization of two distinct allyl pyrophosphatase activities from rat liver microsomes. |
1994 |
Arch. Biochem. Biophys. |
pmid:7986083
|
| Mookhtiar KA et al. |
Yeast squalene synthase. A mechanism for addition of substrates and activation by NADPH. |
1994 |
J. Biol. Chem. |
pmid:8157649
|
| Sagami H et al. |
Novel isoprenoid modified proteins in Halobacteria. |
1994 |
Biochem. Biophys. Res. Commun. |
pmid:8093082
|
| Bradfute DL and Simoni RD |
Non-sterol compounds that regulate cholesterogenesis. Analogues of farnesyl pyrophosphate reduce 3-hydroxy-3-methylglutaryl-coenzyme A reductase levels. |
1994 |
J. Biol. Chem. |
pmid:8120018
|
| Christensen DJ and Poulter CD |
Enzymatic synthesis of isotopically labeled isoprenoid diphosphates. |
1994 |
Bioorg. Med. Chem. |
pmid:7858969
|
| Correll CC et al. |
Identification of farnesol as the non-sterol derivative of mevalonic acid required for the accelerated degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. |
1994 |
J. Biol. Chem. |
pmid:8021239
|
| Zhao J et al. |
Farnesylation of p21 Ras proteins in Xenopus oocytes. |
1994 |
Cell. Mol. Biol. Res. |
pmid:7866432
|
| Sagami H et al. |
Biosynthesis of prenyl diphosphates by cell-free extracts from mammalian tissues. |
1993 |
J. Biochem. |
pmid:8407862
|
| Sagami H et al. |
Geranylgeranyl diphosphate synthase catalyzing the single condensation between isopentenyl diphosphate and farnesyl diphosphate. |
1993 |
J. Biochem. |
pmid:8407863
|
| Ericsson J et al. |
Biosynthesis of dolichol and cholesterol in rat liver peroxisomes. |
1993 |
Biochimie |
pmid:8507678
|
| Omer CA et al. |
Characterization of recombinant human farnesyl-protein transferase: cloning, expression, farnesyl diphosphate binding, and functional homology with yeast prenyl-protein transferases. |
1993 |
Biochemistry |
pmid:8494894
|
| Cane DE et al. |
Overproduction of soluble trichodiene synthase from Fusarium sporotrichioides in Escherichia coli. |
1993 |
Arch. Biochem. Biophys. |
pmid:8424673
|
| Pompliano DL et al. |
Steady-state kinetic mechanism of Ras farnesyl:protein transferase. |
1992 |
Biochemistry |
pmid:1567835
|
| Shechter I et al. |
Solubilization, purification, and characterization of a truncated form of rat hepatic squalene synthetase. |
1992 |
J. Biol. Chem. |
pmid:1569107
|
| Ericsson J et al. |
Isoprenoid biosynthesis in rat liver peroxisomes. Characterization of cis-prenyltransferase and squalene synthetase. |
1992 |
J. Biol. Chem. |
pmid:1527001
|
| Fenton RG et al. |
Regulation of intracellular actin polymerization by prenylated cellular proteins. |
1992 |
J. Cell Biol. |
pmid:1560030
|