| MeSH term | MeSH ID | Detail |
|---|---|---|
| Protozoan Infections | D011528 | 6 associated lipids |
| Leukemia-Lymphoma, Adult T-Cell | D015459 | 25 associated lipids |
| Endometriosis | D004715 | 29 associated lipids |
| Leukemia, Erythroblastic, Acute | D004915 | 41 associated lipids |
| Liver Neoplasms, Experimental | D008114 | 46 associated lipids |
| Osteosarcoma | D012516 | 50 associated lipids |
| Leukemia, Myeloid | D007951 | 52 associated lipids |
| Hypercholesterolemia | D006937 | 91 associated lipids |
| Colonic Neoplasms | D003110 | 161 associated lipids |
| Adenocarcinoma | D000230 | 166 associated lipids |
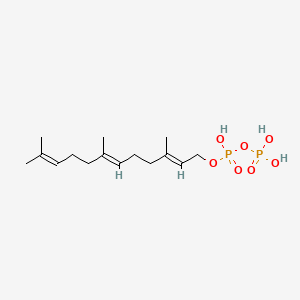
Farnesyl diphosphate
Farnesyl diphosphate is a lipid of Prenol Lipids (PR) class. Farnesyl diphosphate is associated with abnormalities such as Dental caries and Hyperostosis, Diffuse Idiopathic Skeletal. The involved functions are known as Regulation, Process, Signal, Anabolism and inhibitors. Farnesyl diphosphate often locates in peroxisome, Cytoplasmic matrix, Plasma membrane, soluble and Mitochondria. The associated genes with Farnesyl diphosphate are HSD3B1 gene, ABRA gene, MATN1 gene, SEPSECS gene and MBD2 gene. The related lipids are Sterols, 22-hydroxycholesterol, dehydrosqualene, SK&F 104976 and 25-hydroxycholesterol.
Cross Reference
Introduction
To understand associated biological information of Farnesyl diphosphate, we collected biological information of abnormalities, associated pathways, cellular/molecular locations, biological functions, related genes/proteins, lipids and common seen animal/experimental models with organized paragraphs from literatures.
What diseases are associated with Farnesyl diphosphate?
Farnesyl diphosphate is suspected in and other diseases in descending order of the highest number of associated sentences.
Related references are mostly published in these journals:
| Disease | Cross reference | Weighted score | Related literature |
|---|
Possible diseases from mapped MeSH terms on references
We collected disease MeSH terms mapped to the references associated with Farnesyl diphosphate
PubChem Associated disorders and diseases
What pathways are associated with Farnesyl diphosphate
Lipid pathways are not clear in current pathway databases. We organized associated pathways with Farnesyl diphosphate through full-text articles, including metabolic pathways or pathways of biological mechanisms.
Related references are published most in these journals:
| Pathway name | Related literatures |
|---|
PubChem Biomolecular Interactions and Pathways
Link to PubChem Biomolecular Interactions and PathwaysWhat cellular locations are associated with Farnesyl diphosphate?
Visualization in cellular structure
Associated locations are in red color. Not associated locations are in black.
Related references are published most in these journals:
| Location | Cross reference | Weighted score | Related literatures |
|---|
What functions are associated with Farnesyl diphosphate?
Related references are published most in these journals:
| Function | Cross reference | Weighted score | Related literatures |
|---|
What lipids are associated with Farnesyl diphosphate?
Related references are published most in these journals:
| Lipid concept | Cross reference | Weighted score | Related literatures |
|---|
What genes are associated with Farnesyl diphosphate?
Related references are published most in these journals:
| Gene | Cross reference | Weighted score | Related literatures |
|---|
What common seen animal models are associated with Farnesyl diphosphate?
There are no associated biomedical information in the current reference collection.
NCBI Entrez Crosslinks
All references with Farnesyl diphosphate
Download all related citations| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Epand RM et al. | Lipid-mediated a-factor interactions with artificial membranes. | 1995 | Meth. Enzymol. | pmid:7651149 |
| Reiss Y | Substrate interactions of protein prenyltransferases. | 1995 | Meth. Enzymol. | pmid:7651152 |
| Caplin BE and Marshall MS | Mutagenesis and biochemical analysis of recombinant yeast prenyltransferases. | 1995 | Meth. Enzymol. | pmid:7651175 |
| Cohen LH et al. | Different analogues of farnesyl pyrophosphate inhibit squalene synthase and protein:farnesyltransferase to different extents. | 1995 | Biochem. Pharmacol. | pmid:7702642 |
| Lindsey S and Harwood HJ | Inhibition of mammalian squalene synthetase activity by zaragozic acid A is a result of competitive inhibition followed by mechanism-based irreversible inactivation. | 1995 | J. Biol. Chem. | pmid:7721822 |
| Tanaka Y et al. | Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. | 1995 | Nature | pmid:7753173 |
| Furfine ES et al. | Protein farnesyltransferase: kinetics of farnesyl pyrophosphate binding and product release. | 1995 | Biochemistry | pmid:7756316 |
| Dolence JM and Poulter CD | A mechanism for posttranslational modifications of proteins by yeast protein farnesyltransferase. | 1995 | Proc. Natl. Acad. Sci. U.S.A. | pmid:7761439 |
| Harwood HJ | Protein farnesyltransferase: measurement of enzymatic activity in 96-well format using TopCount microplate scintillation counting technology. | 1995 | Anal. Biochem. | pmid:7793628 |
| Thelin A et al. | Effect of squalestatin 1 on the biosynthesis of the mevalonate pathway lipids. | 1994 | Biochim. Biophys. Acta | pmid:7811707 |
| Danesi R et al. | Specific labeling of isoprenylated proteins: application to study inhibitors of the post-translational farnesylation and geranylgeranylation. | 1995 | Biochem. Biophys. Res. Commun. | pmid:7826382 |
| Christensen DJ and Poulter CD | Enzymatic synthesis of isotopically labeled isoprenoid diphosphates. | 1994 | Bioorg. Med. Chem. | pmid:7858969 |
| Soltis DA et al. | Expression, purification, and characterization of the human squalene synthase: use of yeast and baculoviral systems. | 1995 | Arch. Biochem. Biophys. | pmid:7864626 |
| Zhao J et al. | Farnesylation of p21 Ras proteins in Xenopus oocytes. | 1994 | Cell. Mol. Biol. Res. | pmid:7866432 |
| Cane DE et al. | Trichodiene synthase. Substrate specificity and inhibition. | 1995 | Biochemistry | pmid:7873526 |
| Cane DE et al. | Trichodiene synthase. Identification of active site residues by site-directed mutagenesis. | 1995 | Biochemistry | pmid:7873527 |
| Bansal VS and Vaidya S | Characterization of two distinct allyl pyrophosphatase activities from rat liver microsomes. | 1994 | Arch. Biochem. Biophys. | pmid:7986083 |
| Correll CC et al. | Identification of farnesol as the non-sterol derivative of mevalonic acid required for the accelerated degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. | 1994 | J. Biol. Chem. | pmid:8021239 |
| Sagami H et al. | Novel isoprenoid modified proteins in Halobacteria. | 1994 | Biochem. Biophys. Res. Commun. | pmid:8093082 |
| Bradfute DL and Simoni RD | Non-sterol compounds that regulate cholesterogenesis. Analogues of farnesyl pyrophosphate reduce 3-hydroxy-3-methylglutaryl-coenzyme A reductase levels. | 1994 | J. Biol. Chem. | pmid:8120018 |