| MeSH term | MeSH ID | Detail |
|---|---|---|
| Adenocarcinoma | D000230 | 166 associated lipids |
| Colonic Neoplasms | D003110 | 161 associated lipids |
| Endometriosis | D004715 | 29 associated lipids |
| Leukemia, Erythroblastic, Acute | D004915 | 41 associated lipids |
| Hypercholesterolemia | D006937 | 91 associated lipids |
| Leukemia, Myeloid | D007951 | 52 associated lipids |
| Liver Neoplasms, Experimental | D008114 | 46 associated lipids |
| Protozoan Infections | D011528 | 6 associated lipids |
| Osteosarcoma | D012516 | 50 associated lipids |
| Leukemia-Lymphoma, Adult T-Cell | D015459 | 25 associated lipids |
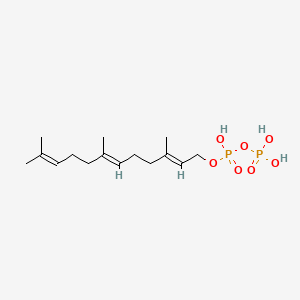
Farnesyl diphosphate
Farnesyl diphosphate is a lipid of Prenol Lipids (PR) class. Farnesyl diphosphate is associated with abnormalities such as Dental caries and Hyperostosis, Diffuse Idiopathic Skeletal. The involved functions are known as Regulation, Process, Signal, Anabolism and inhibitors. Farnesyl diphosphate often locates in peroxisome, Cytoplasmic matrix, Plasma membrane, soluble and Mitochondria. The associated genes with Farnesyl diphosphate are HSD3B1 gene, ABRA gene, MATN1 gene, SEPSECS gene and MBD2 gene. The related lipids are Sterols, 22-hydroxycholesterol, dehydrosqualene, SK&F 104976 and 25-hydroxycholesterol.
Cross Reference
Introduction
To understand associated biological information of Farnesyl diphosphate, we collected biological information of abnormalities, associated pathways, cellular/molecular locations, biological functions, related genes/proteins, lipids and common seen animal/experimental models with organized paragraphs from literatures.
What diseases are associated with Farnesyl diphosphate?
Farnesyl diphosphate is suspected in and other diseases in descending order of the highest number of associated sentences.
Related references are mostly published in these journals:
| Disease | Cross reference | Weighted score | Related literature |
|---|
Possible diseases from mapped MeSH terms on references
We collected disease MeSH terms mapped to the references associated with Farnesyl diphosphate
PubChem Associated disorders and diseases
What pathways are associated with Farnesyl diphosphate
Lipid pathways are not clear in current pathway databases. We organized associated pathways with Farnesyl diphosphate through full-text articles, including metabolic pathways or pathways of biological mechanisms.
Related references are published most in these journals:
| Pathway name | Related literatures |
|---|
PubChem Biomolecular Interactions and Pathways
Link to PubChem Biomolecular Interactions and PathwaysWhat cellular locations are associated with Farnesyl diphosphate?
Visualization in cellular structure
Associated locations are in red color. Not associated locations are in black.
Related references are published most in these journals:
| Location | Cross reference | Weighted score | Related literatures |
|---|
What functions are associated with Farnesyl diphosphate?
Related references are published most in these journals:
| Function | Cross reference | Weighted score | Related literatures |
|---|
What lipids are associated with Farnesyl diphosphate?
Related references are published most in these journals:
| Lipid concept | Cross reference | Weighted score | Related literatures |
|---|
What genes are associated with Farnesyl diphosphate?
Related references are published most in these journals:
| Gene | Cross reference | Weighted score | Related literatures |
|---|
What common seen animal models are associated with Farnesyl diphosphate?
There are no associated biomedical information in the current reference collection.
NCBI Entrez Crosslinks
All references with Farnesyl diphosphate
Download all related citations| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Micali E et al. | Protein farnesyltransferase isoprenoid substrate discrimination is dependent on isoprene double bonds and branched methyl groups. | 2001 | Biochemistry | pmid:11591144 |
| Dursina B et al. | Interaction of yeast Rab geranylgeranyl transferase with its protein and lipid substrates. | 2002 | Biochemistry | pmid:12022885 |
| Holstein SA et al. | Isoprenoids influence expression of Ras and Ras-related proteins. | 2002 | Biochemistry | pmid:12427032 |
| Thomä NH et al. | Phosphoisoprenoid binding specificity of geranylgeranyltransferase type II. | 2000 | Biochemistry | pmid:11009619 |
| Chang SY et al. | Identification of the active conformation and the importance of length of the flexible loop 72-83 in regulating the conformational change of undecaprenyl pyrophosphate synthase. | 2003 | Biochemistry | pmid:14661956 |
| Chen M et al. | Mechanistic insights from the binding of substrate and carbocation intermediate analogues to aristolochene synthase. | 2013 | Biochemistry | pmid:23905850 |
| van der Kamp MW et al. | Conformational change and ligand binding in the aristolochene synthase catalytic cycle. | 2013 | Biochemistry | pmid:24106830 |
| Butrynski JE et al. | Differential isoprenylation of carboxy-terminal mutants of an inhibitory G-protein alpha-subunit: neither farnesylation nor geranylgeranylation is sufficient for membrane attachment. | 1992 | Biochemistry | pmid:1510988 |
| Cane DE et al. | Trichodiene synthase. Probing the role of the highly conserved aspartate-rich region by site-directed mutagenesis. | 1996 | Biochemistry | pmid:8823172 |
| Omer CA et al. | Characterization of recombinant human farnesyl-protein transferase: cloning, expression, farnesyl diphosphate binding, and functional homology with yeast prenyl-protein transferases. | 1993 | Biochemistry | pmid:8494894 |
| Vogt A et al. | Burkitt lymphoma Daudi cells contain two distinct farnesyltransferases with different divalent cation requirements. | 1995 | Biochemistry | pmid:7547984 |
| Cui G and Merz KM | Computational studies of the farnesyltransferase ternary complex part II: the conformational activation of farnesyldiphosphate. | 2007 | Biochemistry | pmid:17918965 |
| Kurth DD et al. | Functional consequence of mutating conserved residues of the yeast farnesyl-protein transferase beta-subunit Ram1(Dpr1). | 1997 | Biochemistry | pmid:9398327 |
| Troutman JM et al. | Protein farnesyl transferase target selectivity is dependent upon peptide stimulated product release. | 2007 | Biochemistry | pmid:17877368 |
| Troutman JM et al. | Selective modification of CaaX peptides with ortho-substituted anilinogeranyl lipids by protein farnesyl transferase: competitive substrates and potent inhibitors from a library of farnesyl diphosphate analogues. | 2007 | Biochemistry | pmid:17854205 |
| Dunten P et al. | Protein farnesyltransferase: structure and implications for substrate binding. | 1998 | Biochemistry | pmid:9609683 |
| Fu Z et al. | Biochemical and structural basis for feedback inhibition of mevalonate kinase and isoprenoid metabolism. | 2008 | Biochemistry | pmid:18302342 |
| Raisig A and Sandmann G | Functional properties of diapophytoene and related desaturases of C(30) and C(40) carotenoid biosynthetic pathways. | 2001 | Biochim. Biophys. Acta | pmid:11566453 |
| Koyama T et al. | Substrate specificity of squalene synthetase. | 1980 | Biochim. Biophys. Acta | pmid:7357018 |
| Newman P et al. | Polyisoprenylation of the CAAX motif--an in vitro protein synthesis study. | 1991 | Biochim. Biophys. Acta | pmid:1954230 |