| MeSH term | MeSH ID | Detail |
|---|---|---|
| Adenocarcinoma | D000230 | 166 associated lipids |
| Lupus Erythematosus, Systemic | D008180 | 43 associated lipids |
| Lung Neoplasms | D008175 | 171 associated lipids |
| Pancreatic Neoplasms | D010190 | 77 associated lipids |
| Colonic Neoplasms | D003110 | 161 associated lipids |
| Body Weight | D001835 | 333 associated lipids |
| Edema | D004487 | 152 associated lipids |
| Prostatic Neoplasms | D011471 | 126 associated lipids |
| Osteosarcoma | D012516 | 50 associated lipids |
| Glioma | D005910 | 112 associated lipids |
| Cell Transformation, Neoplastic | D002471 | 126 associated lipids |
| Thyroid Neoplasms | D013964 | 33 associated lipids |
| Carcinoma, Hepatocellular | D006528 | 140 associated lipids |
| Fibrosis | D005355 | 23 associated lipids |
| Leukemia, Myeloid, Acute | D015470 | 19 associated lipids |
| Hypergammaglobulinemia | D006942 | 9 associated lipids |
| Glioblastoma | D005909 | 27 associated lipids |
| Nephritis | D009393 | 19 associated lipids |
| HIV Infections | D015658 | 20 associated lipids |
| Neurilemmoma | D009442 | 10 associated lipids |
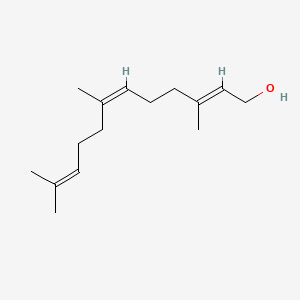
(e,z)-farnesol
(e,z)-farnesol is a lipid of Prenol Lipids (PR) class.
Cross Reference
There are no associated biomedical information in the current reference collection.
Current reference collection contains 3613 references associated with (e,z)-farnesol in LipidPedia. Due to lack of full text of references or no associated biomedical terms are recognized in our current text-mining method, we cannot extract any biomedical terms related to diseases, pathways, locations, functions, genes, lipids, and animal models from the associated reference collection.
Users can download the reference list at the bottom of this page and read the reference manually to find out biomedical information.
Here are additional resources we collected from PubChem and MeSH for (e,z)-farnesol
Possible diseases from mapped MeSH terms on references
We collected disease MeSH terms mapped to the references associated with (e,z)-farnesol
PubChem Biomolecular Interactions and Pathways
All references with (e,z)-farnesol
Download all related citations| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Cox AD et al. | The CAAX peptidomimetic compound B581 specifically blocks farnesylated, but not geranylgeranylated or myristylated, oncogenic ras signaling and transformation. | 1994 | J. Biol. Chem. | pmid:8034681 |
| Gibbs JB et al. | Selective inhibition of farnesyl-protein transferase blocks ras processing in vivo. | 1993 | J. Biol. Chem. | pmid:8463291 |
| Foster JM et al. | Biosynthesis of isoprenoid compounds in Schistosoma mansoni. | 1993 | Mol. Biochem. Parasitol. | pmid:8264731 |
| Inoue H et al. | Formation of farnesal and 3-hydroxy-2,3-dihydrofarnesal from farnesol by protoplasts of Botryococcus braunii. | 1993 | Biochem. Biophys. Res. Commun. | pmid:8250896 |
| Corsini A et al. | Relationship between mevalonate pathway and arterial myocyte proliferation: in vitro studies with inhibitors of HMG-CoA reductase. | 1993 | Atherosclerosis | pmid:8216498 |
| Voziyan PA et al. | Farnesol inhibits phosphatidylcholine biosynthesis in cultured cells by decreasing cholinephosphotransferase activity. | 1993 | Biochem. J. | pmid:8240288 |
| Touhara K and Prestwich GD | Juvenile hormone epoxide hydrolase. Photoaffinity labeling, purification, and characterization from tobacco hornworm eggs. | 1993 | J. Biol. Chem. | pmid:8396141 |
| O'Donnell MP et al. | Lovastatin inhibits proliferation of rat mesangial cells. | 1993 | J. Clin. Invest. | pmid:8423236 |
| Bradfute DL et al. | Squalene synthase-deficient mutant of Chinese hamster ovary cells. | 1992 | J. Biol. Chem. | pmid:1526971 |
| Okazaki S et al. | [A 13-week subcutaneous toxicity study of prednisolone farnesylate (PNF) in rats]. | 1992 | J Toxicol Sci | pmid:1293320 |
| Nagashima Y et al. | [A 13-week percutaneous toxicity study of prednisolone farnesylate (PNF) gel in beagle dogs with a recovery period of 5 weeks]. | 1992 | J Toxicol Sci | pmid:1293321 |
| Nagashima Y et al. | [A 52-week percutaneous toxicity study of prednisolone farnesylate (PNF) gel in beagle dogs with a recovery period of 8 weeks]. | 1992 | J Toxicol Sci | pmid:1293322 |
| Taniguchi H et al. | [Reproductive and developmental toxicity study of prednisolone farnesylate (PNF)--study by subcutaneous administration of PNF prior to and in the early stages of pregnancy in rats]. | 1992 | J Toxicol Sci | pmid:1293323 |
| Taniguchi H et al. | [Reproductive and developmental toxicity study of prednisolone farnesylate (PNF)--study by subcutaneous administration of PNF during the period of fetal organogenesis in rats]. | 1992 | J Toxicol Sci | pmid:1293324 |
| Aso S et al. | [Reproductive and developmental toxicity study of prednisolone farnesylate (PNF)--teratogenicity study in rabbits by subcutaneous administration]. | 1992 | J Toxicol Sci | pmid:1293325 |
| Taniguchi H et al. | [Reproductive and developmental toxicity study of prednisolone farnesylate (PNF)--study of subcutaneous administration of PNF during the perinatal and lactation periods in rats]. | 1992 | J Toxicol Sci | pmid:1293326 |
| Otsuka M et al. | [Mutagenicity studies of prednisolone farnesylate (PNF)]. | 1992 | J Toxicol Sci | pmid:1293327 |
| Uchiyama H et al. | [Effects of prednisolone farnesylate (PNF) gel on skin and ocular mucosa]. | 1992 | J Toxicol Sci | pmid:1293328 |
| Okazaki S et al. | [A 13-week dermal toxicity study of prednisolone farnesylate (PNF) gel in rats with a recovery period of 5 weeks]. | 1992 | J Toxicol Sci | pmid:1293330 |
| Okazaki S et al. | [A 52-week dermal toxicity study of prednisolone farnesylate (PNF) gel in rats with a recovery period of 8 weeks]. | 1992 | J Toxicol Sci | pmid:1293331 |