| MeSH term | MeSH ID | Detail |
|---|---|---|
| Lupus Erythematosus, Systemic | D008180 | 43 associated lipids |
| Osteosarcoma | D012516 | 50 associated lipids |
| Pancreatic Neoplasms | D010190 | 77 associated lipids |
| Insulin Resistance | D007333 | 99 associated lipids |
| Glioma | D005910 | 112 associated lipids |
| Prostatic Neoplasms | D011471 | 126 associated lipids |
| Cell Transformation, Neoplastic | D002471 | 126 associated lipids |
| Carcinoma, Hepatocellular | D006528 | 140 associated lipids |
| Edema | D004487 | 152 associated lipids |
| Colonic Neoplasms | D003110 | 161 associated lipids |
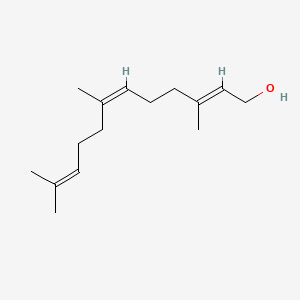
(e,z)-farnesol
(e,z)-farnesol is a lipid of Prenol Lipids (PR) class.
Cross Reference
There are no associated biomedical information in the current reference collection.
Current reference collection contains 3613 references associated with (e,z)-farnesol in LipidPedia. Due to lack of full text of references or no associated biomedical terms are recognized in our current text-mining method, we cannot extract any biomedical terms related to diseases, pathways, locations, functions, genes, lipids, and animal models from the associated reference collection.
Users can download the reference list at the bottom of this page and read the reference manually to find out biomedical information.
Here are additional resources we collected from PubChem and MeSH for (e,z)-farnesol
Possible diseases from mapped MeSH terms on references
We collected disease MeSH terms mapped to the references associated with (e,z)-farnesol
PubChem Biomolecular Interactions and Pathways
All references with (e,z)-farnesol
Download all related citations| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Slakey LL et al. | Occurrence of the enzymes effecting the conversion of acetyl CoA to squalene in homogenates of hog aorta. | 1973 | J. Lipid Res. | pmid:4351784 |
| Beytia E et al. | Squalene synthetase. 3. Mechanism of the reaction. | 1973 | J. Biol. Chem. | pmid:4348553 |
| Qureshi AA et al. | Squalene synthetase. II. Purification and properties of bakers' yeast enzyme. | 1973 | J. Biol. Chem. | pmid:4348552 |
| Heintz R et al. | Plant sterol metabolism. Demonstration and identification of a biosynthetic intermediate between farnesyl PP and squalene in a higher plant. | 1972 | Biochem. Biophys. Res. Commun. | pmid:4344813 |
| Qureshi AA et al. | Squalene synthetase. I. Dissociation and reassociation of enzyme complex. | 1972 | Biochem. Biophys. Res. Commun. | pmid:4341049 |
| Stone KJ and Strominger JL | Inhibition of sterol biosynthesis by bacitracin. | 1972 | Proc. Natl. Acad. Sci. U.S.A. | pmid:4338587 |
| Polito A et al. | Artificial substrates in squalene and sterol biosynthesis. | 1972 | J. Biol. Chem. | pmid:4337856 |
| Slakey LL et al. | The effects of fasting, refeeding, and time of day on the levels of enzymes effecting the conversion of -hydroxy- -methylglutaryl-coenzyme A to squalene. | 1972 | J. Biol. Chem. | pmid:4337504 |
| Kurokawa T et al. | Formation of polyprenyl phosphates by a cell-free enzyme of Micrococcus lysodeikticus. | 1971 | Biochem. Biophys. Res. Commun. | pmid:4334524 |
| George-Nascimento C et al. | Non enzymic formation of nerolidol from farnesyl pyrophosphate in the presence of bivalent cations. | 1971 | Biochem. Biophys. Res. Commun. | pmid:4334520 |
| Castelli A et al. | Effect of hydroxy analogs of coenzyme Q on DPNH- and succin-oxidase activities of yeast mitochondria. | 1971 | Biochem. Biophys. Res. Commun. | pmid:4324835 |
| Dialameh GH et al. | Enzymatic alkylation of menaquinone-o to menaquinones microsomes from chick liver. | 1970 | Biochim. Biophys. Acta | pmid:4323518 |
| Flint AP | The activity and kinetic properties of mevalonate kinase in superovulated rat ovary. | 1970 | Biochem. J. | pmid:4321929 |
| Gough DP and Hemming FW | The characterization and stereochemistry of biosynthesis of dolichols in rat liver. | 1970 | Biochem. J. | pmid:4319540 |
| Heinstein PF et al. | Biosynthesis of gossypol. Incorporation of mevalonate-2-14C and isoprenyl pyrophosphates. | 1970 | J. Biol. Chem. | pmid:4318479 |
| Epstein WW and Rilling HC | Studies on the mechanism of squalene biosynthesis. The structure of presqualene pyrophosphate. | 1970 | J. Biol. Chem. | pmid:4318477 |
| Christenson JG et al. | Enzymatic synthesis of the antigen carrier lipid. | 1969 | J. Biol. Chem. | pmid:4311788 |
| Popják G et al. | Artificial substrates for prenyltransferase. | 1969 | Biochem. J. | pmid:4309597 |
| Sofer SS and Rilling HC | Mechanism of squalene biosynthesis: evidence against the involvement of free nerolidyl pyrophosphate. | 1969 | J. Lipid Res. | pmid:4305712 |
| Popják G et al. | Synthesis of 10,11-dihydrofarnesyl pyrophosphate from 6,7-dihydrogeranyl pyrophosphate by prenyltransferase. | 1969 | Biochem. J. | pmid:4304159 |
| Dorsey JK and Porter JW | The inhibition of mevalonic kinase by geranyl and farnesyl pyrophosphates. | 1968 | J. Biol. Chem. | pmid:4300840 |
| Jungalwala FB and Porter JW | Biosynthesis of phytoene from isopentenyl and farnesyl pyrophosphates by a partially purified tomato enzyme system. | 1967 | Arch. Biochem. Biophys. | pmid:4293186 |
| Holloway PW and Popják G | The purification of 3,3-dimethylallyl- and geranyl-transferase and of isopentenyl pyrophosphate isomerase from pig liver. | 1967 | Biochem. J. | pmid:4292002 |
| Allen CM et al. | A long chain terpenyl pyrophosphate synthetase from Micrococcus lysodeikticus. | 1967 | J. Biol. Chem. | pmid:4290445 |
| Cornforth JW et al. | Studies on the biosynthesis of cholesterol. XX. Steric course of decarboxylation of 5-pyrophosphomevalonate and of the carbon to carbon bond formation in the biosynthesis of farnesyl pyrophosphate. | 1966 | J. Biol. Chem. | pmid:4288360 |
| Rilling HC | A new intermediate in the biosynthesis of squalene. | 1966 | J. Biol. Chem. | pmid:4287912 |
| Benedict CR et al. | Properties of farnesyl pyrophosphate synthetase of pig liver. | 1965 | Arch. Biochem. Biophys. | pmid:4284635 |
| Krishna G et al. | An enzyme-bound intermediate in the conversion of farnesyl pyrophosphate to squalene. | 1964 | Biochem. Biophys. Res. Commun. | pmid:4284347 |
| Muscio F et al. | Prequalene pyrophosphate. A normal intermediate in squalene biosynthesis. | 1974 | J. Biol. Chem. | pmid:4152099 |
| Qureshi AA et al. | Biosynthesis of prelycopersene pyrophosphate and lycopersene by squalene synthetase. | 1973 | J. Biol. Chem. | pmid:4144543 |
| Beedle AS et al. | Studies on the biosynthesis of tetrahymanol in Tetrahymena pyriformis. The mechanism of inhibition by cholesterol. | 1974 | Biochem. J. | pmid:4140721 |
| de Ropp JS and Troy FA | 2H NMR investigation of the organization and dynamics of polyisoprenols in membranes. | 1985 | J. Biol. Chem. | pmid:4066690 |
| Baba T et al. | Dehydrodolichyl diphosphate synthetase from rat seminiferous tubules. | 1987 | Arch. Biochem. Biophys. | pmid:3813545 |
| Gonzalez-Pacanowska D et al. | Isopentenoid synthesis in isolated embryonic Drosophila cells. Farnesol catabolism and omega-oxidation. | 1988 | J. Biol. Chem. | pmid:3335546 |
| Rocha GR et al. | Effect of tt-farnesol and myricetin on in vitro biofilm formed by Streptococcus mutans and Candida albicans. | 2018 | BMC Complement Altern Med | pmid:29444673 |
| pmid:29380558 | ||||
| pmid:29181599 | ||||
| pmid:28684169 | ||||
| pmid:28622460 | ||||
| pmid:28603849 | ||||
| pmid:28584159 | ||||
| pmid:28471040 | ||||
| Cagliero C et al. | Analysis of essential oils and fragrances with a new generation of highly inert gas chromatographic columns coated with ionic liquids. | 2017 | J Chromatogr A | pmid:28343686 |
| Å piÄáková A et al. | Nerolidol and Farnesol Inhibit Some Cytochrome P450 Activities but Did Not Affect Other Xenobiotic-Metabolizing Enzymes in Rat and Human Hepatic Subcellular Fractions. | 2017 | Molecules | pmid:28338641 |
| pmid:28329720 | ||||
| Xia J et al. | In vitro inhibitory effects of farnesol and interactions between farnesol and antifungals against biofilms of Candida albicans resistant strains. | 2017 | Biofouling | pmid:28317391 |
| pmid:28285658 | ||||
| pmid:28284180 | ||||
| pmid:28192189 | ||||
| Wu L et al. | Farnesylthiosalicylic acid sensitizes hepatocarcinoma cells to artemisinin derivatives. | 2017 | PLoS ONE | pmid:28182780 |