| MeSH term | MeSH ID | Detail |
|---|---|---|
| Adenocarcinoma | D000230 | 166 associated lipids |
| Body Weight | D001835 | 333 associated lipids |
| Candidiasis, Oral | D002180 | 11 associated lipids |
| Cell Transformation, Neoplastic | D002471 | 126 associated lipids |
| Colonic Neoplasms | D003110 | 161 associated lipids |
| Edema | D004487 | 152 associated lipids |
| Fibrosis | D005355 | 23 associated lipids |
| Glioblastoma | D005909 | 27 associated lipids |
| Glioma | D005910 | 112 associated lipids |
| Carcinoma, Hepatocellular | D006528 | 140 associated lipids |
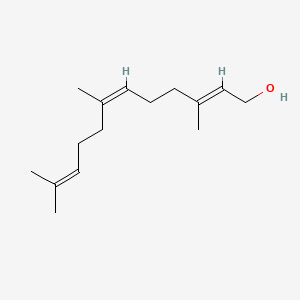
(e,z)-farnesol
(e,z)-farnesol is a lipid of Prenol Lipids (PR) class.
Cross Reference
There are no associated biomedical information in the current reference collection.
Current reference collection contains 3613 references associated with (e,z)-farnesol in LipidPedia. Due to lack of full text of references or no associated biomedical terms are recognized in our current text-mining method, we cannot extract any biomedical terms related to diseases, pathways, locations, functions, genes, lipids, and animal models from the associated reference collection.
Users can download the reference list at the bottom of this page and read the reference manually to find out biomedical information.
Here are additional resources we collected from PubChem and MeSH for (e,z)-farnesol
Possible diseases from mapped MeSH terms on references
We collected disease MeSH terms mapped to the references associated with (e,z)-farnesol
PubChem Biomolecular Interactions and Pathways
All references with (e,z)-farnesol
Download all related citations| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Richards JB and Hemming FW | Dolichols, ubiquinones, geranylgeraniol and farnesol as the major metabolites of mevalonate in Phytophthora cactorum. | 1972 | Biochem. J. | pmid:4643705 |
| Rosado JA and Sage SO | Farnesylcysteine analogues inhibit store-regulated Ca2+ entry in human platelets: evidence for involvement of small GTP-binding proteins and actin cytoskeleton. | 2000 | Biochem. J. | pmid:10727417 |
| Ruiz-Velasco N et al. | Statins upregulate CD36 expression in human monocytes, an effect strengthened when combined with PPAR-gamma ligands Putative contribution of Rho GTPases in statin-induced CD36 expression. | 2004 | Biochem. Pharmacol. | pmid:14698043 |
| Pando R et al. | Ras inhibition attenuates myocardial ischemia-reperfusion injury. | 2009 | Biochem. Pharmacol. | pmid:19426696 |
| Kothapalli R et al. | Effects of long-chain fatty amines on the growth of ras-transformed NIH 3T3 cells. | 1994 | Biochem. Pharmacol. | pmid:8204109 |
| Räikkönen J et al. | Mevalonate pathway intermediates downregulate zoledronic acid-induced isopentenyl pyrophosphate and ATP analog formation in human breast cancer cells. | 2010 | Biochem. Pharmacol. | pmid:19819230 |
| Joo JH et al. | Farnesol activates the intrinsic pathway of apoptosis and the ATF4-ATF3-CHOP cascade of ER stress in human T lymphoblastic leukemia Molt4 cells. | 2015 | Biochem. Pharmacol. | pmid:26275811 |
| Ferri N et al. | Isothiazole dioxide derivative 6n inhibits vascular smooth muscle cell proliferation and protein farnesylation. | 2005 | Biochem. Pharmacol. | pmid:16257390 |
| Erlich S et al. | Ras inhibition results in growth arrest and death of androgen-dependent and androgen-independent prostate cancer cells. | 2006 | Biochem. Pharmacol. | pmid:16780807 |
| Hartmann MA et al. | Metabolism of farnesyl diphosphate in tobacco BY-2 cells treated with squalestatin. | 2000 | Biochem. Soc. Trans. | pmid:11171211 |
| Dinamarco TM et al. | Farnesol-induced cell death in the filamentous fungus Aspergillus nidulans. | 2011 | Biochem. Soc. Trans. | pmid:21936849 |
| Lowe PN et al. | Expression of polyisoprenylated Ras proteins in the insect/baculovirus system. | 1992 | Biochem. Soc. Trans. | pmid:1397645 |
| Nigg EA et al. | Targeting lamin proteins to the nuclear envelope: the role of CaaX box modifications. | 1992 | Biochem. Soc. Trans. | pmid:1397650 |
| Haklai R et al. | Dislodgment and accelerated degradation of Ras. | 1998 | Biochemistry | pmid:9477957 |
| Strickland CL et al. | Crystal structure of farnesyl protein transferase complexed with a CaaX peptide and farnesyl diphosphate analogue. | 1998 | Biochemistry | pmid:9843427 |
| Nishimori R et al. | Biosynthesis of unnatural bacteriochlorophyll c derivatives esterified with α,ω-diols in the green sulfur photosynthetic bacterium Chlorobaculum tepidum. | 2011 | Biochemistry | pmid:21846125 |
| Clausen VA et al. | Stereochemical analysis of the reaction catalyzed by human protein geranylgeranyl transferase. | 2001 | Biochemistry | pmid:11300771 |
| Murataliev MB et al. | Chimeragenesis of the fatty acid binding site of cytochrome P450BM3. Replacement of residues 73-84 with the homologous residues from the insect cytochrome P450 CYP4C7. | 2004 | Biochemistry | pmid:14967018 |
| Koyama T et al. | Identification of significant residues in the substrate binding site of Bacillus stearothermophilus farnesyl diphosphate synthase. | 1996 | Biochemistry | pmid:8755734 |
| Busch S and Unruh T | The influence of additives on the nanoscopic dynamics of the phospholipid dimyristoylphosphatidylcholine. | 2011 | Biochim. Biophys. Acta | pmid:21036141 |
| Koyama T et al. | Substrate specificity of squalene synthetase. | 1980 | Biochim. Biophys. Acta | pmid:7357018 |
| Parker TS et al. | Inhibition of liver prenyltransferase by alkyl phosphonates and phosphonophosphates. | 1978 | Biochim. Biophys. Acta | pmid:687653 |
| Dialameh GH et al. | Enzymatic alkylation of menaquinone-o to menaquinones microsomes from chick liver. | 1970 | Biochim. Biophys. Acta | pmid:4323518 |
| Aharonson Z et al. | Stringent structural requirements for anti-Ras activity of S-prenyl analogues. | 1998 | Biochim. Biophys. Acta | pmid:9545527 |
| Ghosh PM et al. | Lovastatin induces apoptosis by inhibiting mitotic and post-mitotic events in cultured mesangial cells. | 1997 | Biochim. Biophys. Acta | pmid:9398081 |
| Rowat AC and Davis JH | Farnesol-DMPC phase behaviour: a (2)H-NMR study. | 2004 | Biochim. Biophys. Acta | pmid:15003880 |
| Shechter I | Biosynthesis of trans-farnesyl triphosphate in Gibberella fujikuroi. | 1973 | Biochim. Biophys. Acta | pmid:4795388 |
| Hecht SM | Mass spectrometric identification of some prenylaminopurines. | 1970 | Biochim. Biophys. Acta | pmid:5507911 |
| Shechter I | Phosphate transfer from trans-farnesyl triphosphate to AMP in Gibberella fujikuroi. | 1974 | Biochim. Biophys. Acta | pmid:4423368 |
| Elad G et al. | Targeting of K-Ras 4B by S-trans,trans-farnesyl thiosalicylic acid. | 1999 | Biochim. Biophys. Acta | pmid:10590312 |
| Bertolino A et al. | Polyisoprenoid amphiphilic compounds as inhibitors of squalene synthesis and other microsomal enzymes. | 1978 | Biochim. Biophys. Acta | pmid:210830 |
| Rowat AC et al. | Effects of farnesol on the physical properties of DMPC membranes. | 2005 | Biochim. Biophys. Acta | pmid:15963943 |
| Haug JS et al. | Directed cell killing (apoptosis) in human lymphoblastoid cells incubated in the presence of farnesol: effect of phosphatidylcholine. | 1994 | Biochim. Biophys. Acta | pmid:8061045 |
| DeBarber AE et al. | Omega-hydroxylation of farnesol by mammalian cytochromes p450. | 2004 | Biochim. Biophys. Acta | pmid:15158752 |
| Umetani N et al. | Lovastatin inhibits gene expression of type-I scavenger receptor in THP-1 human macrophages. | 1996 | Biochim. Biophys. Acta | pmid:8908154 |
| Bifulco M et al. | Inhibition of farnesylation blocks growth but not differentiation in FRTL-5 thyroid cells. | 1999 | Biochimie | pmid:10401660 |
| Samuel O et al. | Preparation of (1-3H) polyprenyl pyrophosphates. | 1974 | Biochimie | pmid:4375498 |
| Zhang X et al. | PEG-farnesylthiosalicylate conjugate as a nanomicellar carrier for delivery of paclitaxel. | 2013 | Bioconjug. Chem. | pmid:23425093 |
| Zhang X et al. | Reduction-sensitive dual functional nanomicelles for improved delivery of paclitaxel. | 2014 | Bioconjug. Chem. | pmid:25121577 |
| Xia J et al. | In vitro inhibitory effects of farnesol and interactions between farnesol and antifungals against biofilms of Candida albicans resistant strains. | 2017 | Biofouling | pmid:28317391 |
| Inoue Y et al. | Farnesol-Induced Disruption of the Staphylococcus aureus Cytoplasmic Membrane. | 2016 | Biol. Pharm. Bull. | pmid:27150138 |
| Sato T et al. | Farnesol, a morphogenetic autoregulatory substance in the dimorphic fungus Candida albicans, inhibits hyphae growth through suppression of a mitogen-activated protein kinase cascade. | 2004 | Biol. Pharm. Bull. | pmid:15133261 |
| Bhattacharyya S et al. | Sol-gel silica controlled release thin films for the inhibition of methicillin-resistant Staphylococcus aureus. | 2014 | Biomaterials | pmid:24099711 |
| Xie J et al. | Microparticles developed by electrohydrodynamic atomization for the local delivery of anticancer drug to treat C6 glioma in vitro. | 2006 | Biomaterials | pmid:16490248 |
| Abdel-Rhman SH et al. | Effect of Tyrosol and Farnesol on Virulence and Antibiotic Resistance of Clinical Isolates of Pseudomonas aeruginosa. | 2015 | Biomed Res Int | pmid:26844228 |
| Ling Y et al. | Synthesis and evaluation of nitric oxide-releasing derivatives of farnesylthiosalicylic acid as anti-tumor agents. | 2010 | Bioorg. Med. Chem. | pmid:20435479 |
| Ling Y et al. | Synthesis and biological evaluation of farnesylthiosalicylamides as potential anti-tumor agents. | 2014 | Bioorg. Med. Chem. | pmid:24300920 |
| Shchepin R et al. | Influence of heterocyclic and oxime-containing farnesol analogs on quorum sensing and pathogenicity in Candida albicans. | 2008 | Bioorg. Med. Chem. | pmid:18037299 |
| Endo S et al. | Chromene-3-carboxamide derivatives discovered from virtual screening as potent inhibitors of the tumour maker, AKR1B10. | 2010 | Bioorg. Med. Chem. | pmid:20304656 |
| Khalil AA et al. | Isolation and characterization of a monoamine oxidase B selective inhibitor from tobacco smoke. | 2006 | Bioorg. Med. Chem. | pmid:16458520 |
| Tsuji F et al. | The geranyl-modified tryptophan residue is crucial for ComXRO-E-2 pheromone biological activity. | 2011 | Bioorg. Med. Chem. Lett. | pmid:21636272 |
| Bhagatji P et al. | Multiple cellular proteins modulate the dynamics of K-ras association with the plasma membrane. | 2010 | Biophys. J. | pmid:21081081 |
| Beedle AM and Zamponi GW | Block of voltage-dependent calcium channels by aliphatic monoamines. | 2000 | Biophys. J. | pmid:10866952 |
| Nunes PM et al. | Study of trans-trans farnesol effect on hyphae formation by Yarrowia lipolytica. | 2013 | Bioprocess Biosyst Eng | pmid:23715764 |
| Rahman NK et al. | Enzymatic synthesis of farnesyl laurate in organic solvent: initial water activity, kinetics mechanism, optimization of continuous operation using packed bed reactor and mass transfer studies. | 2011 | Bioprocess Biosyst Eng | pmid:21327986 |
| Ohto C et al. | Prenyl alcohol production by expression of exogenous isopentenyl diphosphate isomerase and farnesyl diphosphate synthase genes in Escherichia coli. | 2009 | Biosci. Biotechnol. Biochem. | pmid:19129660 |
| Nagai H et al. | Development of a novel PPARγ ligand screening system using pinpoint fluorescence-probed protein. | 2011 | Biosci. Biotechnol. Biochem. | pmid:21307572 |
| Wang C et al. | Farnesol production from Escherichia coli by harnessing the exogenous mevalonate pathway. | 2010 | Biotechnol. Bioeng. | pmid:20552672 |
| Song L | Recovery of E,E-farnesol from cultures of yeast erg9 mutants: extraction with polymeric beads and purification by normal-phase chromatography. | 2009 Jul-Aug | Biotechnol. Prog. | pmid:19569196 |
| Nasr N et al. | HIV-1 infection of human macrophages directly induces viperin which inhibits viral production. | 2012 | Blood | pmid:22677126 |
| Rocha GR et al. | Effect of tt-farnesol and myricetin on in vitro biofilm formed by Streptococcus mutans and Candida albicans. | 2018 | BMC Complement Altern Med | pmid:29444673 |
| Jeon JG et al. | Influences of naturally occurring agents in combination with fluoride on gene expression and structural organization of Streptococcus mutans in biofilms. | 2009 | BMC Microbiol. | pmid:19863808 |
| Cerca N et al. | Confocal laser scanning microscopy analysis of S. epidermidis biofilms exposed to farnesol, vancomycin and rifampicin. | 2012 | BMC Res Notes | pmid:22591918 |
| Langman MJ et al. | Treatment of chronic gastric ulcer with carbenoxolone and gefarnate: a comparative trial. | 1973 | Br Med J | pmid:4577839 |
| Alves FR et al. | Antibiofilm and antibacterial activities of farnesol and xylitol as potential endodontic irrigants. | 2013 | Braz Dent J | pmid:23969910 |
| Alves FR et al. | Biofilm biomass disruption by natural substances with potential for endodontic use. | 2013 Jan-Feb | Braz Oral Res | pmid:23306623 |
| Journe F et al. | Farnesol, a mevalonate pathway intermediate, stimulates MCF-7 breast cancer cell growth through farnesoid-X-receptor-mediated estrogen receptor activation. | 2008 | Breast Cancer Res. Treat. | pmid:17333335 |
| Still K et al. | Effects of risedronate, alendronate, and etidronate on the viability and activity of rat bone marrow stromal cells in vitro. | 2003 | Calcif. Tissue Int. | pmid:12457261 |
| Braun PC | The effect of farnesol on amino acid incorporation by a wild-type and cell-wall variant strain of Candida albicans. | 2005 | Can. J. Microbiol. | pmid:16234870 |
| Tsimberidou AM et al. | Phase 1 first-in-human clinical study of S-trans,trans-farnesylthiosalicylic acid (salirasib) in patients with solid tumors. | 2010 | Cancer Chemother. Pharmacol. | pmid:19484470 |
| Haklai R et al. | Orally administered FTS (salirasib) inhibits human pancreatic tumor growth in nude mice. | 2008 | Cancer Chemother. Pharmacol. | pmid:17909812 |
| Au-Yeung KK et al. | Herbal isoprenols induce apoptosis in human colon cancer cells through transcriptional activation of PPARgamma. | 2008 | Cancer Invest. | pmid:18608213 |
| Joo JH and Jetten AM | Molecular mechanisms involved in farnesol-induced apoptosis. | 2010 | Cancer Lett. | pmid:19520495 |
| Mo H et al. | Farnesyl anthranilate suppresses the growth, in vitro and in vivo, of murine B16 melanomas. | 2000 | Cancer Lett. | pmid:10936674 |
| Yazlovitskaya EM and Melnykovych G | Selective farnesol toxicity and translocation of protein kinase C in neoplastic HeLa-S3K and non-neoplastic CF-3 cells. | 1995 | Cancer Lett. | pmid:7874691 |
| Adany I et al. | Differences in sensitivity to farnesol toxicity between neoplastically- and non-neoplastically-derived cells in culture. | 1994 | Cancer Lett. | pmid:8019976 |
| McAnally JA et al. | Farnesyl-O-acetylhydroquinone and geranyl-O-acetylhydroquinone suppress the proliferation of murine B16 melanoma cells, human prostate and colon adenocarcinoma cells, human lung carcinoma cells, and human leukemia cells. | 2003 | Cancer Lett. | pmid:14643448 |
| Shipman CM et al. | The bisphosphonate incadronate (YM175) causes apoptosis of human myeloma cells in vitro by inhibiting the mevalonate pathway. | 1998 | Cancer Res. | pmid:9850051 |
| Blum R et al. | Gene expression signature of human cancer cell lines treated with the ras inhibitor salirasib (S-farnesylthiosalicylic acid). | 2007 | Cancer Res. | pmid:17409441 |
| Zhang L and Hill RP | Hypoxia enhances metastatic efficiency in HT1080 fibrosarcoma cells by increasing cell survival in lungs, not cell adhesion and invasion. | 2007 | Cancer Res. | pmid:17699784 |
| Joo JH et al. | Farnesol-induced apoptosis in human lung carcinoma cells is coupled to the endoplasmic reticulum stress response. | 2007 | Cancer Res. | pmid:17699800 |
| Blum R et al. | Ras inhibition in glioblastoma down-regulates hypoxia-inducible factor-1alpha, causing glycolysis shutdown and cell death. | 2005 | Cancer Res. | pmid:15705901 |
| Eskens FA et al. | Farnesyl transferase inhibitors: current developments and future perspectives. | 2000 | Cancer Treat. Rev. | pmid:11006134 |
| Ong TP et al. | Farnesol and geraniol chemopreventive activities during the initial phases of hepatocarcinogenesis involve similar actions on cell proliferation and DNA damage, but distinct actions on apoptosis, plasma cholesterol and HMGCoA reductase. | 2006 | Carcinogenesis | pmid:16332721 |
| Szűcs G et al. | Cardioprotection by farnesol: role of the mevalonate pathway. | 2013 | Cardiovasc Drugs Ther | pmid:23673412 |
| Pando R et al. | The Ras antagonist farnesylthiosalicylic acid ameliorates experimental myocarditis in the rat. | 2010 Mar-Apr | Cardiovasc. Pathol. | pmid:19144546 |
| RodrÃguez C et al. | Statins normalize vascular lysyl oxidase down-regulation induced by proatherogenic risk factors. | 2009 | Cardiovasc. Res. | pmid:19406911 |
| Yoo S et al. | Antimicrobial traits of tea- and cranberry-derived polyphenols against Streptococcus mutans. | 2011 | Caries Res. | pmid:21720161 |
| Søgaard M et al. | A rab protein is required for the assembly of SNARE complexes in the docking of transport vesicles. | 1994 | Cell | pmid:7923363 |
| Aronheim A et al. | Membrane targeting of the nucleotide exchange factor Sos is sufficient for activating the Ras signaling pathway. | 1994 | Cell | pmid:7923364 |
| Forman BM et al. | Identification of a nuclear receptor that is activated by farnesol metabolites. | 1995 | Cell | pmid:7774010 |
| Zoccarato F et al. | The adenosine inhibition of glutamate exocytosis in synaptosomes is removed by the collapse of the vesicle-cytosol deltapH plus the opening of farnesol-sensitive Ca(2+) channels. | 2003 | Cell Calcium | pmid:12618148 |
| Shapira S et al. | The tumor suppressor neurofibromin confers sensitivity to apoptosis by Ras-dependent and Ras-independent pathways. | 2007 | Cell Death Differ. | pmid:17096025 |
| Amos S et al. | Farnesylthiosalicylic acid induces caspase activation and apoptosis in glioblastoma cells. | 2006 | Cell Death Differ. | pmid:16239932 |
| Shalom-Feuerstein R et al. | Restoration of sensitivity to anoikis in Ras-transformed rat intestinal epithelial cells by a Ras inhibitor. | 2004 | Cell Death Differ. | pmid:14576773 |
| Goldberg L et al. | FTS and 2-DG induce pancreatic cancer cell death and tumor shrinkage in mice. | 2012 | Cell Death Dis | pmid:22419113 |
| Charette N et al. | Salirasib sensitizes hepatocarcinoma cells to TRAIL-induced apoptosis through DR5 and survivin-dependent mechanisms. | 2013 | Cell Death Dis | pmid:23348585 |
| Makovski V et al. | Analysis of gene expression array in TSC2-deficient AML cells reveals IRF7 as a pivotal factor in the Rheb/mTOR pathway. | 2014 | Cell Death Dis | pmid:25476905 |
| Okamoto S et al. | Zoledronic acid induces apoptosis and S-phase arrest in mesothelioma through inhibiting Rab family proteins and topoisomerase II actions. | 2014 | Cell Death Dis | pmid:25393473 |
| Lee J et al. | Proto-oncogenic H-Ras, K-Ras, and N-Ras are involved in muscle differentiation via phosphatidylinositol 3-kinase. | 2010 | Cell Res. | pmid:20603646 |