| MeSH term | MeSH ID | Detail |
|---|---|---|
| Hemolysis | D006461 | 131 associated lipids |
| Leishmaniasis | D007896 | 19 associated lipids |
| Mycoses | D009181 | 18 associated lipids |
| Tinea | D014005 | 5 associated lipids |
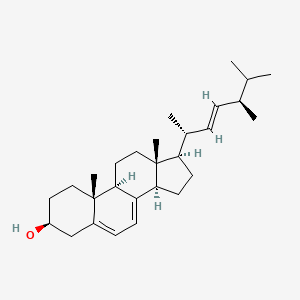
Ergosterol
Ergosterol is a lipid of Sterol Lipids (ST) class. Ergosterol is associated with abnormalities such as Disintegration (morphologic abnormality), Consumption-archaic term for TB, Candidiasis, Mycoses and Iodotyrosyl coupling defect. The involved functions are known as Anabolism, sporulation, 5-(carboxyamino)imidazole ribonucleotide mutase activity, Subtraction process and Physiologic Organization. Ergosterol often locates in Pore, Membrane, Protoplasm, Plasma membrane and Endoplasmic Reticulum. The associated genes with Ergosterol are IMPACT gene, BLVRB gene, CYP51A1 gene, CDR1 wt Allele and HM13 gene. The related lipids are Sterols, Cardiolipins, Membrane Lipids, fecosterol and Phosphatidylserines. The related experimental models are Knock-out.
Cross Reference
Introduction
To understand associated biological information of Ergosterol, we collected biological information of abnormalities, associated pathways, cellular/molecular locations, biological functions, related genes/proteins, lipids and common seen animal/experimental models with organized paragraphs from literatures.
What diseases are associated with Ergosterol?
Ergosterol is suspected in Infection, Mycoses, Candidiasis, Chagas Disease, Cyst, Dermatophytosis and other diseases in descending order of the highest number of associated sentences.
Related references are mostly published in these journals:
| Disease | Cross reference | Weighted score | Related literature |
|---|
Possible diseases from mapped MeSH terms on references
We collected disease MeSH terms mapped to the references associated with Ergosterol
PubChem Associated disorders and diseases
What pathways are associated with Ergosterol
Lipid pathways are not clear in current pathway databases. We organized associated pathways with Ergosterol through full-text articles, including metabolic pathways or pathways of biological mechanisms.
Related references are published most in these journals:
| Pathway name | Related literatures |
|---|
PubChem Biomolecular Interactions and Pathways
Link to PubChem Biomolecular Interactions and PathwaysWhat cellular locations are associated with Ergosterol?
Visualization in cellular structure
Associated locations are in red color. Not associated locations are in black.
Related references are published most in these journals:
| Location | Cross reference | Weighted score | Related literatures |
|---|
What functions are associated with Ergosterol?
Related references are published most in these journals:
| Function | Cross reference | Weighted score | Related literatures |
|---|
What lipids are associated with Ergosterol?
Related references are published most in these journals:
| Lipid concept | Cross reference | Weighted score | Related literatures |
|---|
What genes are associated with Ergosterol?
Related references are published most in these journals:
| Gene | Cross reference | Weighted score | Related literatures |
|---|
What common seen animal models are associated with Ergosterol?
Knock-out
Knock-out are used in the study 'Multidrug transporters CaCdr1p and CaMdr1p of Candida albicans display different lipid specificities: both ergosterol and sphingolipids are essential for targeting of CaCdr1p to membrane rafts.' (Pasrija R et al., 2008) and Knock-out are used in the study 'UPC2A is required for high-level azole antifungal resistance in Candida glabrata.' (Whaley SG et al., 2014).
Related references are published most in these journals:
| Model | Cross reference | Weighted score | Related literatures |
|---|
NCBI Entrez Crosslinks
All references with Ergosterol
Download all related citations| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Mitropoulos KA et al. | Lanosterol 14alpha-demethylase. Similarity of the enzyme system from yeast and rat liver. | 1976 | Steroids | pmid:781918 |
| Sjöstrand U et al. | Minor and trace sterols from marine invertebrates 29. (22E)-ergosta-5,22,25-trien-3 beta-ol and (22E,24R)-24,26-dimethylcholesta-5,22,25(27)-trien-3 beta-ol. Two new marine sterols from the sponge Pseudaxinella lunacharta. | 1981 | Steroids | pmid:7303039 |
| Naumowicz M et al. | Chronopotentiometric studies of phosphatidylcholine bilayers modified by ergosterol. | 2011 Sep-Oct | Steroids | pmid:21641920 |
| Hu JF et al. | 26-Nor-25-isopropyl-ergosta-5,7,22E-trien-3beta-ol: a new C(29) sterol from the sponge Agelas sceptrum from Jamaica. | 2002 | Steroids | pmid:12123785 |
| Sato S et al. | Identification of 23-demethylacanthasterol in an asteroid, Acanthaster planci and its synthesis. | 1980 | Steroids | pmid:6893371 |
| Bortolotto M et al. | Chemical studies of marine inverterbrates. XXIX 4 alpha-Methyl-3 beta 8-beta-dihydroxy-5 alpha-ergost-24(28)-en-23-one, a novel polyoxygenated sterol from the soft coral Litophyton viridis. (Coelenterata, Octocorallia, Alcyonacea). | 1977 | Steroids | pmid:22145 |
| Jeong TM et al. | Sterols from a species of Pichia, a n-alkane-utilizing yeast. | 1975 | Steroids | pmid:1154452 |
| Morisaki M et al. | Nutritional effect of possible intermediates of phytosterol dealkylation in the silkworm, Bombyx mori. | 1974 | Steroids | pmid:4421556 |
| Gebreyesus T et al. | Minor and trace sterols in marine invertebrates 55. The isolation, structure elucidation and synthesis of ergosta-5, 24(28), 25-trien-3 beta-ol. | 1985 | Steroids | pmid:2871644 |
| Brosa C et al. | New synthetic strategy for the synthesis of 24-epibrassinolide. | 1996 | Steroids | pmid:8883220 |
| MacÃas FA et al. | Bioactive steroids from Oryza sativa L. | 2006 | Steroids | pmid:16620896 |
| Zhang Y et al. | Ergosterimide, a new natural Diels-Alder adduct of a steroid and maleimide in the fungus Aspergillus niger. | 2007 | Steroids | pmid:17628622 |
| Harki E et al. | Identification and quantification of Tuber melanosporum Vitt. sterols. | 1996 | Steroids | pmid:8910974 |
| Alcazar-Fuoli L et al. | Ergosterol biosynthesis pathway in Aspergillus fumigatus. | 2008 | Steroids | pmid:18191972 |
| Mehdi H et al. | Sterols of Acanthamoeba culbertsoni strain A-1. | 1988 May-Jun | Steroids | pmid:3242176 |
| Nes WD et al. | Evidence for similarities and differences in the biosynthesis of fungal sterols. | 1989 Mar-May | Steroids | pmid:2678609 |
| Fattorusso E et al. | 4,4-Dimethyl-5 alpha-ergosta-8,24(28)-dien-3 beta-ol from the fungus Marasmius oreades. | 1992 | Steroids | pmid:1621266 |
| Wang F and Liu JK | Two new steryl esters from the basidiomycete Tricholomopsis rutilans. | 2005 | Steroids | pmid:15631869 |
| Fuska J et al. | Microbial transformations of natural antitumor agents. 23. Conversion of withaferin-A to 12 beta- and 15 beta-hydroxy derivatives of withaferin-A. | 1982 | Steroids | pmid:7157453 |
| Stonard RJ et al. | A new C26 sterol peroxide from the opisthobranch mollusk Adalaria sp. and the sea pen Virgularia sp. | 1980 | Steroids | pmid:7414658 |