| MeSH term | MeSH ID | Detail |
|---|---|---|
| Hypercholesterolemia | D006937 | 91 associated lipids |
| Hyperlipoproteinemia Type II | D006938 | 22 associated lipids |
| Hyperlipidemias | D006949 | 73 associated lipids |
| Hyperlipoproteinemias | D006951 | 15 associated lipids |
| Hyperlipoproteinemia Type III | D006952 | 4 associated lipids |
| Hypersensitivity, Delayed | D006968 | 43 associated lipids |
| Inflammation | D007249 | 119 associated lipids |
| Influenza, Human | D007251 | 11 associated lipids |
| Keloid | D007627 | 12 associated lipids |
| Leukemia | D007938 | 74 associated lipids |
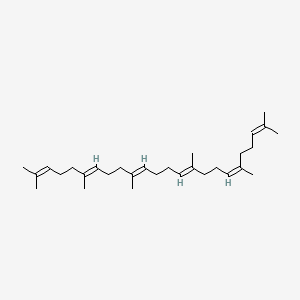
(e,e,e,e)-squalene
(e,e,e,e)-squalene is a lipid of Fatty Acyls (FA) class. (e,e,e,e)-squalene is associated with abnormalities such as Hypercholesterolemia and Cataract. The involved functions are known as Process, metaplastic cell transformation, Protein Overexpression, Anabolism and Biosynthetic Pathways. (e,e,e,e)-squalene often locates in Membrane, Protoplasm, Plasma membrane, Tissue membrane and Back. The associated genes with (e,e,e,e)-squalene are Genome, IMPACT gene, GAPDH gene, GTF2I gene and Chromatin. The related lipids are Membrane Lipids, cycloartenol, Sterols, Fatty Acids and Nonesterified Fatty Acids.
Cross Reference
Introduction
To understand associated biological information of (e,e,e,e)-squalene, we collected biological information of abnormalities, associated pathways, cellular/molecular locations, biological functions, related genes/proteins, lipids and common seen animal/experimental models with organized paragraphs from literatures.
What diseases are associated with (e,e,e,e)-squalene?
(e,e,e,e)-squalene is suspected in Hypercholesterolemia, Cataract and other diseases in descending order of the highest number of associated sentences.
Related references are mostly published in these journals:
| Disease | Cross reference | Weighted score | Related literature |
|---|
Possible diseases from mapped MeSH terms on references
We collected disease MeSH terms mapped to the references associated with (e,e,e,e)-squalene
PubChem Associated disorders and diseases
What pathways are associated with (e,e,e,e)-squalene
There are no associated biomedical information in the current reference collection.
PubChem Biomolecular Interactions and Pathways
Link to PubChem Biomolecular Interactions and PathwaysWhat cellular locations are associated with (e,e,e,e)-squalene?
Visualization in cellular structure
Associated locations are in red color. Not associated locations are in black.
Related references are published most in these journals:
| Location | Cross reference | Weighted score | Related literatures |
|---|
What functions are associated with (e,e,e,e)-squalene?
Related references are published most in these journals:
| Function | Cross reference | Weighted score | Related literatures |
|---|
What lipids are associated with (e,e,e,e)-squalene?
Related references are published most in these journals:
| Lipid concept | Cross reference | Weighted score | Related literatures |
|---|
What genes are associated with (e,e,e,e)-squalene?
Related references are published most in these journals:
| Gene | Cross reference | Weighted score | Related literatures |
|---|
What common seen animal models are associated with (e,e,e,e)-squalene?
There are no associated biomedical information in the current reference collection.
NCBI Entrez Crosslinks
All references with (e,e,e,e)-squalene
Download all related citations| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Rasch MR et al. | Chains, sheets, and droplets: assemblies of hydrophobic gold nanocrystals with saturated phosphatidylcholine lipid and squalene. | 2012 | Langmuir | pmid:23033891 |
| Singh M et al. | MF59 oil-in-water emulsion in combination with a synthetic TLR4 agonist (E6020) is a potent adjuvant for a combination Meningococcus vaccine. | 2012 | Hum Vaccin Immunother | pmid:22832252 |
| Vesikari T et al. | Assessment of squalene adjuvanted and non-adjuvanted vaccines against pandemic H1N1 influenza in children 6 months to 17 years of age. | 2012 | Hum Vaccin Immunother | pmid:22906943 |
| Della Cioppa G et al. | Superior immunogenicity of seasonal influenza vaccines containing full dose of MF59 (®) adjuvant: results from a dose-finding clinical trial in older adults. | 2012 | Hum Vaccin Immunother | pmid:22426371 |
| Faenzi E et al. | One dose of an MF59-adjuvanted pandemic A/H1N1 vaccine recruits pre-existing immune memory and induces the rapid rise of neutralizing antibodies. | 2012 | Vaccine | pmid:22521851 |
| Hatz C et al. | A randomised, single-blind, dose-range study to assess the immunogenicity and safety of a cell-culture-derived A/H1N1 influenza vaccine in adult and elderly populations. | 2012 | Vaccine | pmid:22626675 |
| Hwang SM et al. | Comparison of the adverse events associated with MF59-adjuvanted and non-adjuvanted H1N1 vaccines in healthy young male Korean soldiers. | 2012 | Jpn. J. Infect. Dis. | pmid:22627298 |
| Siegrist CA et al. | Responses of solid organ transplant recipients to the AS03-adjuvanted pandemic influenza vaccine. | 2012 | Antivir. Ther. (Lond.) | pmid:22544169 |
| Yang M et al. | MF59 formulated with CpG ODN as a potent adjuvant of recombinant HSP65-MUC1 for inducing anti-MUC1+ tumor immunity in mice. | 2012 | Int. Immunopharmacol. | pmid:22595192 |
| Cabrera-Vique C et al. | Bioactive compounds and nutritional significance of virgin argan oil--an edible oil with potential as a functional food. | 2012 | Nutr. Rev. | pmid:22537213 |
| Stella B et al. | Nonpolymeric nanoassemblies for ocular administration of acyclovir: pharmacokinetic evaluation in rabbits. | 2012 | Eur J Pharm Biopharm | pmid:22008147 |
| Oral E et al. | A new mechanism of oxidation in ultrahigh molecular weight polyethylene caused by squalene absorption. | 2012 | J. Biomed. Mater. Res. Part B Appl. Biomater. | pmid:22190411 |
| Sancho A et al. | More on influenza vaccine in young children. | 2012 | N. Engl. J. Med. | pmid:22738111 |
| Yang P et al. | Two concise enantioselective total syntheses of (-)-glabrescol implicate alternative biosynthetic pathways starting from squalene. | 2012 | Org. Lett. | pmid:22817611 |
| RamÃrez-Torres A et al. | Proteomics and gene expression analyses of squalene-supplemented mice identify microsomal thioredoxin domain-containing protein 5 changes associated with hepatic steatosis. | 2012 | J Proteomics | pmid:22796066 |
| Surls J et al. | Increased membrane cholesterol in lymphocytes diverts T-cells toward an inflammatory response. | 2012 | PLoS ONE | pmid:22723880 |
| Girod A et al. | Composition of fingermark residue: a qualitative and quantitative review. | 2012 | Forensic Sci. Int. | pmid:22727572 |
| Sarpietro MG et al. | Squalenoyl prodrug of paclitaxel: synthesis and evaluation of its incorporation in phospholipid bilayers. | 2012 | Int J Pharm | pmid:22728161 |
| Wu S et al. | Engineering triterpene metabolism in tobacco. | 2012 | Planta | pmid:22729821 |
| Langley JM et al. | Randomized, multicenter trial of a single dose of AS03-adjuvanted or unadjuvanted H1N1 2009 pandemic influenza vaccine in children 6 months to <9 years of age: safety and immunogenicity. | 2012 | Pediatr. Infect. Dis. J. | pmid:22801094 |
| Block SL et al. | Dose-range study of MF59-adjuvanted versus nonadjuvanted monovalent A/H1N1 pandemic influenza vaccine in six- to less than thirty-six-month-old children. | 2012 | Pediatr. Infect. Dis. J. | pmid:22481427 |
| Sémiramoth N et al. | Self-assembled squalenoylated penicillin bioconjugates: an original approach for the treatment of intracellular infections. | 2012 | ACS Nano | pmid:22482704 |
| Langley JM et al. | Safety and immunogenicity of 2010-2011 H1N12009-containing trivalent inactivated influenza vaccine in children 12-59 months of age previously given AS03-adjuvanted H1N12009 pandemic vaccine: a PHAC/CIHR Influenza Research Network (PCIRN) study. | 2012 | Vaccine | pmid:22469860 |
| Dell'Era L et al. | Immunogenicity, safety and tolerability of MF59-adjuvanted seasonal influenza vaccine in children with juvenile idiopathic arthritis. | 2012 | Vaccine | pmid:22138210 |
| Allain V et al. | Self-assembled nucleolipids: from supramolecular structure to soft nucleic acid and drug delivery devices. | 2012 | Nucleic Acids Res. | pmid:22075995 |
| Garçon N et al. | Development and evaluation of AS03, an Adjuvant System containing α-tocopherol and squalene in an oil-in-water emulsion. | 2012 | Expert Rev Vaccines | pmid:22380826 |
| RamÃrez-Torres A et al. | Proteomics and gene expression analyses of mitochondria from squalene-treated apoE-deficient mice identify short-chain specific acyl-CoA dehydrogenase changes associated with fatty liver amelioration. | 2012 | J Proteomics | pmid:22402057 |
| Tronchoni J et al. | Lipid composition of wine strains of Saccharomyces kudriavzevii and Saccharomyces cerevisiae grown at low temperature. | 2012 | Int. J. Food Microbiol. | pmid:22405355 |
| Fox CB et al. | Immunomodulatory and physical effects of phospholipid composition in vaccine adjuvant emulsions. | 2012 | AAPS PharmSciTech | pmid:22415641 |
| Nassim C et al. | Identification of antigen and adjuvant doses resulting in optimal immunogenicity and antibody persistence up to 1 year after immunization with a pandemic A/H1N1 influenza vaccine in children 3 to < 9 years of age. | 2012 | Pediatr. Infect. Dis. J. | pmid:22418661 |
| Fox CB et al. | Effects on immunogenicity by formulations of emulsion-based adjuvants for malaria vaccines. | 2012 | Clin. Vaccine Immunol. | pmid:22896687 |
| Reynales H et al. | A prospective observational safety study on MF59(®) adjuvanted cell culture-derived vaccine, Celtura(®) during the A/H1N1 (2009) influenza pandemic. | 2012 | Vaccine | pmid:22902681 |
| Naziri E et al. | Recovery of squalene from wine lees using ultrasound assisted extraction-a feasibility study. | 2012 | J. Agric. Food Chem. | pmid:22888984 |
| Dorea JG | Safety of thimerosal in vaccines: for whom and how many doses? | 2011 Mar-Apr | Therapie | pmid:23189333 |
| Kozlov VG and Viktorova EG | [Evaluation of the efficiency and reactogenicity of emulsion-based adjuvant systems in the manufacture of polyclonal enteroviral diagnostic sera]. | 2011 Mar-Apr | Vopr. Virusol. | pmid:21545042 |
| Durando P et al. | Adjuvants and alternative routes of administration towards the development of the ideal influenza vaccine. | 2011 Jan-Feb | Hum Vaccin | pmid:21245655 |
| Schellenberger MT et al. | Evaluation of adjuvants for a candidate conjugate vaccine against benzo[a]pyrene. | 2011 Jan-Feb | Hum Vaccin | pmid:21245662 |
| Sarpietro MG et al. | Synthesis of n-squalenoyl cytarabine and evaluation of its affinity with phospholipid bilayers and monolayers. | 2011 | Int J Pharm | pmid:21219999 |
| Brasser AJ et al. | Presence of wax esters and squalene in human saliva. | 2011 | Arch. Oral Biol. | pmid:21247555 |
| Arguedas A et al. | Assessment of the safety, tolerability and kinetics of the immune response to A/H1N1v vaccine formulations with and without adjuvant in healthy pediatric subjects from 3 through 17 years of age. | 2011 | Hum Vaccin | pmid:21285531 |
| Pariani E et al. | Response to 2009 pandemic and seasonal influenza vaccines co-administered to HIV-infected and HIV-uninfected former drug users living in a rehabilitation community in Italy. | 2011 | Vaccine | pmid:21974995 |
| Andrews NJ et al. | Predictors of immune response and reactogenicity to AS03B-adjuvanted split virion and non-adjuvanted whole virion H1N1 (2009) pandemic influenza vaccines. | 2011 | Vaccine | pmid:21875635 |
| Vesikari T et al. | Oil-in-water emulsion adjuvant with influenza vaccine in young children. | 2011 | N. Engl. J. Med. | pmid:21995388 |
| Oliaro-Bosso S et al. | Characterization of the channel constriction allowing the access of the substrate to the active site of yeast oxidosqualene cyclase. | 2011 | PLoS ONE | pmid:21811565 |
| Bildstein L et al. | Interaction of an amphiphilic squalenoyl prodrug of gemcitabine with cellular membranes. | 2011 | Eur J Pharm Biopharm | pmid:21784150 |
| Della Cioppa G et al. | Trivalent and quadrivalent MF59(®)-adjuvanted influenza vaccine in young children: a dose- and schedule-finding study. | 2011 | Vaccine | pmid:21906647 |
| Tabache F et al. | Acute polyarthritis after influenza A (H1N1) immunization. | 2011 | Joint Bone Spine | pmid:21444232 |
| Sun Y et al. | A reproducible in-vivo model of lymphatic malformation in rats. | 2011 | J. Comp. Pathol. | pmid:21419420 |
| Meier S et al. | Antibody responses to natural influenza A/H1N1/09 disease or following immunization with adjuvanted vaccines, in immunocompetent and immunocompromised children. | 2011 | Vaccine | pmid:21419775 |
| Réjiba S et al. | Squalenoyl gemcitabine nanomedicine overcomes the low efficacy of gemcitabine therapy in pancreatic cancer. | 2011 | Nanomedicine | pmid:21419876 |