| MeSH term | MeSH ID | Detail |
|---|---|---|
| Demyelinating Diseases | D003711 | 15 associated lipids |
| Hyperlipoproteinemias | D006951 | 15 associated lipids |
| Rosacea | D012393 | 13 associated lipids |
| Keloid | D007627 | 12 associated lipids |
| Otitis Media | D010033 | 12 associated lipids |
| Dermatitis, Occupational | D009783 | 12 associated lipids |
| Influenza, Human | D007251 | 11 associated lipids |
| Hepatitis | D006505 | 11 associated lipids |
| Pregnancy Complications, Infectious | D011251 | 11 associated lipids |
| Scalp Dermatoses | D012536 | 11 associated lipids |
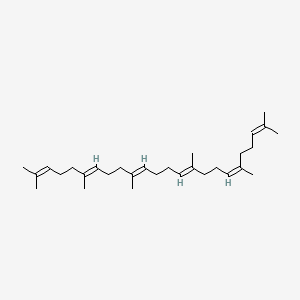
(e,e,e,e)-squalene
(e,e,e,e)-squalene is a lipid of Fatty Acyls (FA) class. (e,e,e,e)-squalene is associated with abnormalities such as Hypercholesterolemia and Cataract. The involved functions are known as Process, metaplastic cell transformation, Protein Overexpression, Anabolism and Biosynthetic Pathways. (e,e,e,e)-squalene often locates in Membrane, Protoplasm, Plasma membrane, Tissue membrane and Back. The associated genes with (e,e,e,e)-squalene are Genome, IMPACT gene, GAPDH gene, GTF2I gene and Chromatin. The related lipids are Membrane Lipids, cycloartenol, Sterols, Fatty Acids and Nonesterified Fatty Acids.
Cross Reference
Introduction
To understand associated biological information of (e,e,e,e)-squalene, we collected biological information of abnormalities, associated pathways, cellular/molecular locations, biological functions, related genes/proteins, lipids and common seen animal/experimental models with organized paragraphs from literatures.
What diseases are associated with (e,e,e,e)-squalene?
(e,e,e,e)-squalene is suspected in Hypercholesterolemia, Cataract and other diseases in descending order of the highest number of associated sentences.
Related references are mostly published in these journals:
| Disease | Cross reference | Weighted score | Related literature |
|---|
Possible diseases from mapped MeSH terms on references
We collected disease MeSH terms mapped to the references associated with (e,e,e,e)-squalene
PubChem Associated disorders and diseases
What pathways are associated with (e,e,e,e)-squalene
There are no associated biomedical information in the current reference collection.
PubChem Biomolecular Interactions and Pathways
Link to PubChem Biomolecular Interactions and PathwaysWhat cellular locations are associated with (e,e,e,e)-squalene?
Visualization in cellular structure
Associated locations are in red color. Not associated locations are in black.
Related references are published most in these journals:
| Location | Cross reference | Weighted score | Related literatures |
|---|
What functions are associated with (e,e,e,e)-squalene?
Related references are published most in these journals:
| Function | Cross reference | Weighted score | Related literatures |
|---|
What lipids are associated with (e,e,e,e)-squalene?
Related references are published most in these journals:
| Lipid concept | Cross reference | Weighted score | Related literatures |
|---|
What genes are associated with (e,e,e,e)-squalene?
Related references are published most in these journals:
| Gene | Cross reference | Weighted score | Related literatures |
|---|
What common seen animal models are associated with (e,e,e,e)-squalene?
There are no associated biomedical information in the current reference collection.
NCBI Entrez Crosslinks
All references with (e,e,e,e)-squalene
Download all related citations| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Barchfeld GL et al. | The adjuvants MF59 and LT-K63 enhance the mucosal and systemic immunogenicity of subunit influenza vaccine administered intranasally in mice. | 1999 | Vaccine | pmid:10067675 |
| Dupuis M et al. | Distribution of adjuvant MF59 and antigen gD2 after intramuscular injection in mice. | 1999 | Vaccine | pmid:10519932 |
| Stephenson I et al. | Boosting immunity to influenza H5N1 with MF59-adjuvanted H5N3 A/Duck/Singapore/97 vaccine in a primed human population. | 2003 | Vaccine | pmid:12639491 |
| Romera SA et al. | Adjuvant effects of sulfolipo-cyclodextrin in a squalane-in-water and water-in-mineral oil emulsions for BHV-1 vaccines in cattle. | 2000 | Vaccine | pmid:10924795 |
| Boyce TG et al. | Safety and immunogenicity of adjuvanted and unadjuvanted subunit influenza vaccines administered intranasally to healthy adults. | 2000 | Vaccine | pmid:10930676 |
| Süli J et al. | Experimental squalene adjuvant. I. Preparation and testing of its effectiveness. | 2004 | Vaccine | pmid:15308373 |
| BenÃsek Z et al. | Experimental squalene adjuvant. II. Harmlessness and local reactogenity. | 2004 | Vaccine | pmid:15308374 |
| Cristiani C et al. | Safety of MF-59 adjuvanted vaccine for pandemic influenza: results of the vaccination campaign in an Italian health district. | 2011 | Vaccine | pmid:21396903 |
| Morel S et al. | Adjuvant System AS03 containing α-tocopherol modulates innate immune response and leads to improved adaptive immunity. | 2011 | Vaccine | pmid:21256188 |
| Keitel W et al. | Dose ranging of adjuvant and antigen in a cell culture H5N1 influenza vaccine: safety and immunogenicity of a phase 1/2 clinical trial. | 2010 | Vaccine | pmid:19835829 |
| Vesikari T et al. | MF59-adjuvanted influenza vaccine (FLUAD) in children: safety and immunogenicity following a second year seasonal vaccination. | 2009 | Vaccine | pmid:19840662 |
| Okike IO et al. | The immunogenicity of a novel A (H1N1) vaccine in HIV-infected children. | 2011 | Vaccine | pmid:21742005 |
| de Roux A et al. | Impact of corticosteroids on the immune response to a MF59-adjuvanted influenza vaccine in elderly COPD-patients. | 2006 | Vaccine | pmid:16288937 |
| Precioso AR et al. | A phase I randomized, double-blind, controlled trial of 2009 influenza A (H1N1) inactivated monovalent vaccines with different adjuvant systems. | 2011 | Vaccine | pmid:21945258 |
| Candela S et al. | An early (3-6 weeks) active surveillance study to assess the safety of pandemic influenza vaccine Focetria in a province of Emilia-Romagna region, Italy - part one. | 2013 | Vaccine | pmid:22766247 |
| Langley JM et al. | Safety and immunogenicity of 2010-2011 H1N12009-containing trivalent inactivated influenza vaccine in children 12-59 months of age previously given AS03-adjuvanted H1N12009 pandemic vaccine: a PHAC/CIHR Influenza Research Network (PCIRN) study. | 2012 | Vaccine | pmid:22469860 |
| Fukase H et al. | Assessment of the immunogenicity and safety of varying doses of an MF59®-adjuvanted cell culture-derived A/H1N1 pandemic influenza vaccine in Japanese paediatric, adult and elderly subjects. | 2012 | Vaccine | pmid:22472791 |
| Reynales H et al. | A prospective observational safety study on MF59(®) adjuvanted cell culture-derived vaccine, Celtura(®) during the A/H1N1 (2009) influenza pandemic. | 2012 | Vaccine | pmid:22902681 |
| Ventura R et al. | Technology transfer of an oil-in-water vaccine-adjuvant for strengthening pandemic influenza preparedness in Indonesia. | 2013 | Vaccine | pmid:22884665 |
| Moro ML et al. | A population based cohort study to assess the safety of pandemic influenza vaccine Focetria in Emilia-Romagna region, Italy - part two. | 2013 | Vaccine | pmid:22885015 |