| MeSH term | MeSH ID | Detail |
|---|---|---|
| Acne Vulgaris | D000152 | 35 associated lipids |
| Adenocarcinoma | D000230 | 166 associated lipids |
| Alopecia Areata | D000506 | 6 associated lipids |
| Alzheimer Disease | D000544 | 76 associated lipids |
| Olfaction Disorders | D000857 | 17 associated lipids |
| Arteriosclerosis | D001161 | 86 associated lipids |
| Arthritis | D001168 | 41 associated lipids |
| Arthritis, Experimental | D001169 | 24 associated lipids |
| Body Weight | D001835 | 333 associated lipids |
| Brain Diseases | D001927 | 27 associated lipids |
| Celiac Disease | D002446 | 16 associated lipids |
| Cell Transformation, Neoplastic | D002471 | 126 associated lipids |
| Cholelithiasis | D002769 | 16 associated lipids |
| Cholestasis | D002779 | 23 associated lipids |
| Chondrodysplasia Punctata | D002806 | 8 associated lipids |
| Chronic Disease | D002908 | 7 associated lipids |
| Colonic Neoplasms | D003110 | 161 associated lipids |
| Coronary Artery Disease | D003324 | 47 associated lipids |
| Coronary Disease | D003327 | 70 associated lipids |
| Demyelinating Diseases | D003711 | 15 associated lipids |
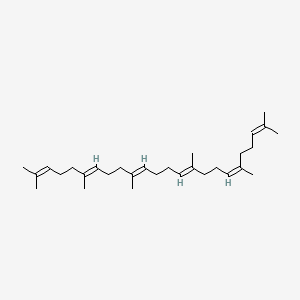
(e,e,e,e)-squalene
(e,e,e,e)-squalene is a lipid of Fatty Acyls (FA) class. (e,e,e,e)-squalene is associated with abnormalities such as Hypercholesterolemia and Cataract. The involved functions are known as Process, metaplastic cell transformation, Protein Overexpression, Anabolism and Biosynthetic Pathways. (e,e,e,e)-squalene often locates in Membrane, Protoplasm, Plasma membrane, Tissue membrane and Back. The associated genes with (e,e,e,e)-squalene are Genome, IMPACT gene, GAPDH gene, GTF2I gene and Chromatin. The related lipids are Membrane Lipids, cycloartenol, Sterols, Fatty Acids and Nonesterified Fatty Acids.
Cross Reference
Introduction
To understand associated biological information of (e,e,e,e)-squalene, we collected biological information of abnormalities, associated pathways, cellular/molecular locations, biological functions, related genes/proteins, lipids and common seen animal/experimental models with organized paragraphs from literatures.
What diseases are associated with (e,e,e,e)-squalene?
(e,e,e,e)-squalene is suspected in Hypercholesterolemia, Cataract and other diseases in descending order of the highest number of associated sentences.
Related references are mostly published in these journals:
| Disease | Cross reference | Weighted score | Related literature |
|---|
Possible diseases from mapped MeSH terms on references
We collected disease MeSH terms mapped to the references associated with (e,e,e,e)-squalene
PubChem Associated disorders and diseases
What pathways are associated with (e,e,e,e)-squalene
There are no associated biomedical information in the current reference collection.
PubChem Biomolecular Interactions and Pathways
Link to PubChem Biomolecular Interactions and PathwaysWhat cellular locations are associated with (e,e,e,e)-squalene?
Visualization in cellular structure
Associated locations are in red color. Not associated locations are in black.
Related references are published most in these journals:
| Location | Cross reference | Weighted score | Related literatures |
|---|
What functions are associated with (e,e,e,e)-squalene?
Related references are published most in these journals:
| Function | Cross reference | Weighted score | Related literatures |
|---|
What lipids are associated with (e,e,e,e)-squalene?
Related references are published most in these journals:
| Lipid concept | Cross reference | Weighted score | Related literatures |
|---|
What genes are associated with (e,e,e,e)-squalene?
Related references are published most in these journals:
| Gene | Cross reference | Weighted score | Related literatures |
|---|
What common seen animal models are associated with (e,e,e,e)-squalene?
There are no associated biomedical information in the current reference collection.
NCBI Entrez Crosslinks
All references with (e,e,e,e)-squalene
Download all related citations| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Ott G et al. | Enhancement of humoral response against human influenza vaccine with the simple submicron oil/water emulsion adjuvant MF59. | 1995 | Vaccine | pmid:8578842 |
| Higgins DA et al. | MF59 adjuvant enhances the immunogenicity of influenza vaccine in both young and old mice. | 1996 | Vaccine | pmid:8782343 |
| Hilgers LA et al. | A novel non-mineral oil-based adjuvant. I. Efficacy of a synthetic sulfolipopolysaccharide in a squalane-in-water emulsion in laboratory animals. | 1994 | Vaccine | pmid:8085385 |
| Hilgers LA et al. | A novel non-mineral oil-based adjuvant. II. Efficacy of a synthetic sulfolipopolysaccharide in a squalane-in-water emulsion in pigs. | 1994 | Vaccine | pmid:8085386 |
| Byars NE et al. | Vaccinating guinea pigs with recombinant glycoprotein D of herpes simplex virus in an efficacious adjuvant formulation elicits protection against vaginal infection. | 1994 | Vaccine | pmid:8165851 |
| Singh M et al. | A comparison of biodegradable microparticles and MF59 as systemic adjuvants for recombinant gD from HSV-2. | 1998 | Vaccine | pmid:9795387 |
| Heineman TC et al. | A randomized, controlled study in adults of the immunogenicity of a novel hepatitis B vaccine containing MF59 adjuvant. | 1999 | Vaccine | pmid:10438046 |
| De Donato S et al. | Safety and immunogenicity of MF59-adjuvanted influenza vaccine in the elderly. | 1999 | Vaccine | pmid:10462245 |
| Minutello M et al. | Safety and immunogenicity of an inactivated subunit influenza virus vaccine combined with MF59 adjuvant emulsion in elderly subjects, immunized for three consecutive influenza seasons. | 1999 | Vaccine | pmid:9987141 |
| Hilgers LA et al. | Sulfolipo-cyclodextrin in squalane-in-water as a novel and safe vaccine adjuvant. | 1999 | Vaccine | pmid:9987157 |
| Podda A | The adjuvanted influenza vaccines with novel adjuvants: experience with the MF59-adjuvanted vaccine. | 2001 | Vaccine | pmid:11257408 |
| Greer CE et al. | The comparison of the effect of LTR72 and MF59 adjuvants on mouse humoral response to intranasal immunisation with human papillomavirus type 6b (HPV-6b) virus-like particles. | 2000 | Vaccine | pmid:11137233 |
| Skeiky YA et al. | Protective efficacy of a tandemly linked, multi-subunit recombinant leishmanial vaccine (Leish-111f) formulated in MPL adjuvant. | 2002 | Vaccine | pmid:12213399 |
| Iorio AM et al. | Antibody responses and HIV-1 viral load in HIV-1-seropositive subjects immunised with either the MF59-adjuvanted influenza vaccine or a conventional non-adjuvanted subunit vaccine during highly active antiretroviral therapy. | 2003 | Vaccine | pmid:12922092 |
| Black S et al. | Safety of MF59-adjuvanted versus non-adjuvanted influenza vaccines in children and adolescents: an integrated analysis. | 2010 | Vaccine | pmid:20813217 |
| Madhun AS et al. | An adjuvanted pandemic influenza H1N1 vaccine provides early and long term protection in health care workers. | 2010 | Vaccine | pmid:21034828 |
| Garcia-Sicilia J et al. | Immunogenicity and safety of AS03-adjuvanted H1N1 pandemic vaccines in children and adolescents. | 2011 | Vaccine | pmid:21504774 |
| Decaro N et al. | Immunogenicity and protective efficacy in dogs of an MF59â„¢-adjuvanted vaccine against recombinant canine/porcine coronavirus. | 2011 | Vaccine | pmid:21272607 |
| Calabro S et al. | Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. | 2011 | Vaccine | pmid:21215831 |
| Javelle E et al. | Delayed focal lipoatrophy after AS03-adjuvanted influenza A (H1N1) 2009 vaccine. | 2011 | Vaccine | pmid:21172376 |
| Esposito S et al. | Pandemic influenza A/H1N1 vaccine administered sequentially or simultaneously with seasonal influenza vaccine to HIV-infected children and adolescents. | 2011 | Vaccine | pmid:21199699 |
| Esposito S et al. | Immunogenicity, safety and tolerability of monovalent 2009 pandemic influenza A/H1N1 MF59-adjuvanted vaccine in patients with β-thalassemia major. | 2010 | Vaccine | pmid:20888873 |
| Pellegrini M et al. | MF59-adjuvanted versus non-adjuvanted influenza vaccines: integrated analysis from a large safety database. | 2009 | Vaccine | pmid:19751689 |
| Forrest HL et al. | Single- and multiple-clade influenza A H5N1 vaccines induce cross protection in ferrets. | 2009 | Vaccine | pmid:19406182 |
| Camilloni B et al. | Cross-reactive antibodies in middle-aged and elderly volunteers after MF59-adjuvanted subunit trivalent influenza vaccine against B viruses of the B/Victoria or B/Yamagata lineages. | 2009 | Vaccine | pmid:19410623 |
| Phillips CJ et al. | Antibodies to squalene in US Navy Persian Gulf War veterans with chronic multisymptom illness. | 2009 | Vaccine | pmid:19379786 |
| Ansaldi F et al. | Antibody response against heterogeneous circulating influenza virus strains elicited by MF59- and non-adjuvanted vaccines during seasons with good or partial matching between vaccine strain and clinical isolates. | 2010 | Vaccine | pmid:20433807 |
| Carmona A et al. | Immunogenicity and safety of AS03-adjuvanted 2009 influenza A H1N1 vaccine in children 6-35 months. | 2010 | Vaccine | pmid:20600478 |
| Frey SE et al. | Safety and immunogenicity of HCV E1E2 vaccine adjuvanted with MF59 administered to healthy adults. | 2010 | Vaccine | pmid:20619382 |
| Camilloni B et al. | An influenza B outbreak during the 2007/2008 winter among appropriately immunized elderly people living in a nursing home. | 2010 | Vaccine | pmid:20846530 |
| Fabbiani M et al. | Immune response to influenza A (H1N1)v monovalent MF59-adjuvanted vaccine in HIV-infected patients. | 2011 | Vaccine | pmid:21349364 |
| Dell'Era L et al. | Immunogenicity, safety and tolerability of MF59-adjuvanted seasonal influenza vaccine in children with juvenile idiopathic arthritis. | 2012 | Vaccine | pmid:22138210 |
| Radosević K et al. | Antibody and T-cell responses to a virosomal adjuvanted H9N2 avian influenza vaccine: impact of distinct additional adjuvants. | 2008 | Vaccine | pmid:18514980 |
| Brito LA et al. | An alternative renewable source of squalene for use in emulsion adjuvants. | 2011 | Vaccine | pmid:21723355 |
| Puig-Barberà J et al. | Effectiveness of MF59-adjuvanted subunit influenza vaccine in preventing hospitalisations for cardiovascular disease, cerebrovascular disease and pneumonia in the elderly. | 2007 | Vaccine | pmid:17889411 |
| Guillon C et al. | Formulation of HIV-1 Tat and p24 antigens by PLA nanoparticles or MF59 impacts the breadth, but not the magnitude, of serum and faecal antibody responses in rabbits. | 2007 | Vaccine | pmid:17904700 |
| de Bruijn I et al. | Antibody induction by virosomal, MF59-adjuvanted, or conventional influenza vaccines in the elderly. | 2007 | Vaccine | pmid:18063446 |
| Wack A et al. | Combination adjuvants for the induction of potent, long-lasting antibody and T-cell responses to influenza vaccine in mice. | 2008 | Vaccine | pmid:18162266 |
| O'Hagan DT et al. | Cationic microparticles are a potent delivery system for a HCV DNA vaccine. | 2004 | Vaccine | pmid:15542189 |
| Schultze V et al. | Safety of MF59 adjuvant. | 2008 | Vaccine | pmid:18462843 |
| O'Hagan DT et al. | The mechanism of action of MF59 - an innately attractive adjuvant formulation. | 2012 | Vaccine | pmid:22682289 |
| Yu S et al. | Novel Th1-biased adjuvant, SPO1, enhances mucosal and systemic immunogenicity of vaccines administered intranasally in mice. | 2012 | Vaccine | pmid:22709954 |
| Dey AK et al. | Use of a polyanionic carbomer, Carbopol971P, in combination with MF59, improves antibody responses to HIV-1 envelope glycoprotein. | 2012 | Vaccine | pmid:22366638 |
| Chandramouli S et al. | Generation of a parvovirus B19 vaccine candidate. | 2013 | Vaccine | pmid:23827313 |
| Yang WH et al. | Long-term immunogenicity of an AS03-adjuvanted influenza A(H1N1)pdm09 vaccine in young and elderly adults: an observer-blind, randomized trial. | 2013 | Vaccine | pmid:23856331 |
| Calabro S et al. | The adjuvant effect of MF59 is due to the oil-in-water emulsion formulation, none of the individual components induce a comparable adjuvant effect. | 2013 | Vaccine | pmid:23684834 |
| Tetsutani K and Ishii KJ | Adjuvants in influenza vaccines. | 2012 | Vaccine | pmid:23084848 |
| Nielsen AB et al. | Immune response after one or two doses of pandemic influenza A (H1N1) monovalent, AS03-adjuvanted vaccine in HIV infected adults. | 2012 | Vaccine | pmid:23036498 |
| Poder A et al. | An observer-blind, randomized, multi-center trial assessing long-term safety and immunogenicity of AS03-adjuvanted or unadjuvanted H1N1/2009 influenza vaccines in children 10-17 years of age. | 2014 | Vaccine | pmid:24252703 |
| Rouleau I et al. | Increased risk of anaphylaxis following administration of 2009 AS03-adjuvanted monovalent pandemic A/H1N1 (H1N1pdm09) vaccine. | 2013 | Vaccine | pmid:24144473 |