| MeSH term | MeSH ID | Detail |
|---|---|---|
| Abscess | D000038 | 13 associated lipids |
| Arthritis | D001168 | 41 associated lipids |
| Body Weight | D001835 | 333 associated lipids |
| Burns | D002056 | 34 associated lipids |
| Edema | D004487 | 152 associated lipids |
| Hypertension, Renovascular | D006978 | 10 associated lipids |
| Psoriasis | D011565 | 47 associated lipids |
| Blood Loss, Surgical | D016063 | 6 associated lipids |
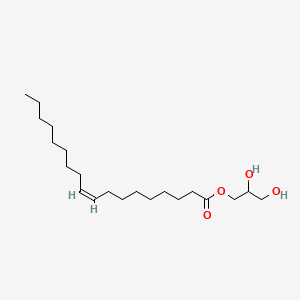
2,3-Dihydroxypropyl oleate
2,3-Dihydroxypropyl oleate is a lipid of Glycerolipids (GL) class. The involved functions are known as enzyme activity and acyltransferase activity. 2,3-dihydroxypropyl oleate often locates in soluble fraction.
Cross Reference
Introduction
To understand associated biological information of 2,3-Dihydroxypropyl oleate, we collected biological information of abnormalities, associated pathways, cellular/molecular locations, biological functions, related genes/proteins, lipids and common seen animal/experimental models with organized paragraphs from literatures.
What diseases are associated with 2,3-Dihydroxypropyl oleate?
There are no associated biomedical information in the current reference collection.
Possible diseases from mapped MeSH terms on references
We collected disease MeSH terms mapped to the references associated with 2,3-Dihydroxypropyl oleate
PubChem Associated disorders and diseases
What pathways are associated with 2,3-Dihydroxypropyl oleate
There are no associated biomedical information in the current reference collection.
PubChem Biomolecular Interactions and Pathways
Link to PubChem Biomolecular Interactions and PathwaysWhat cellular locations are associated with 2,3-Dihydroxypropyl oleate?
Visualization in cellular structure
Associated locations are in red color. Not associated locations are in black.
Related references are published most in these journals:
| Location | Cross reference | Weighted score | Related literatures |
|---|
What functions are associated with 2,3-Dihydroxypropyl oleate?
Related references are published most in these journals:
| Function | Cross reference | Weighted score | Related literatures |
|---|
What lipids are associated with 2,3-Dihydroxypropyl oleate?
There are no associated biomedical information in the current reference collection.
What genes are associated with 2,3-Dihydroxypropyl oleate?
There are no associated biomedical information in the current reference collection.
What common seen animal models are associated with 2,3-Dihydroxypropyl oleate?
There are no associated biomedical information in the current reference collection.
NCBI Entrez Crosslinks
All references with 2,3-Dihydroxypropyl oleate
Download all related citations| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Kwon TK and Kim JC | In vitro skin permeation of monoolein nanoparticles containing hydroxypropyl beta-cyclodextrin/minoxidil complex. | 2010 | Int J Pharm | pmid:20362653 |
| Mulet X et al. | High throughput preparation and characterisation of amphiphilic nanostructured nanoparticulate drug delivery vehicles. | 2010 | Int J Pharm | pmid:20580796 |
| Gan L et al. | Self-assembled liquid crystalline nanoparticles as a novel ophthalmic delivery system for dexamethasone: Improving preocular retention and ocular bioavailability. | 2010 | Int J Pharm | pmid:20558263 |
| Boyd BJ et al. | A lipid-based liquid crystalline matrix that provides sustained release and enhanced oral bioavailability for a model poorly water soluble drug in rats. | 2007 | Int J Pharm | pmid:17467935 |
| Phan S et al. | Evaluating the link between self-assembled mesophase structure and drug release. | 2011 | Int J Pharm | pmid:21963475 |
| Shah MH et al. | Spray dried glyceryl monooleate-magnesium trisilicate dry powder as cubic phase precursor. | 2006 | Int J Pharm | pmid:16846704 |
| Ferreira DA et al. | Cryo-TEM investigation of phase behaviour and aggregate structure in dilute dispersions of monoolein and oleic acid. | 2006 | Int J Pharm | pmid:16439076 |
| Pepe D et al. | Decylglucoside-based microemulsions for cutaneous localization of lycopene and ascorbic acid. | 2012 | Int J Pharm | pmid:22692080 |
| Réeff J et al. | Characterization and optimization of GMO-based gels with long term release for intraarticular administration. | 2013 | Int J Pharm | pmid:23651644 |
| Sato H et al. | Development of cyclosporine A-loaded dry-emulsion formulation using highly purified glycerol monooleate for safe inhalation therapy. | 2013 | Int J Pharm | pmid:23528280 |
| Siekmann B et al. | Preparation and structural investigations of colloidal dispersions prepared from cubic monoglyceride-water phases. | 2002 | Int J Pharm | pmid:12204563 |
| Gonçalves VSS et al. | Application of RPMI 2650 as a cell model to evaluate solid formulations for intranasal delivery of drugs. | 2016 | Int J Pharm | pmid:27702697 |
| Shi C et al. | Exploring the effect of hydrophilic and hydrophobic structure of grafted polymeric micelles on drug loading. | 2016 | Int J Pharm | pmid:27576669 |
| Lee J and Kellaway IW | Buccal permeation of [D-Ala(2), D-Leu(5)]enkephalin from liquid crystalline phases of glyceryl monooleate. | 2000 | Int J Pharm | pmid:10675680 |
| Milak S and Zimmer A | Glycerol monooleate liquid crystalline phases used in drug delivery systems. | 2015 | Int J Pharm | pmid:25479099 |
| Isaksson S et al. | Influence of three alloplastic materials on calvarial bone healing. An experimental evaluation of HTR-polymer, lactomer beads, and a carrier gel. | 1993 | Int J Oral Maxillofac Surg | pmid:8106817 |
| Shen J et al. | Preparation and evaluation of a self-nanoemulsifying drug delivery system loaded with Akebia saponin D-phospholipid complex. | 2016 | Int J Nanomedicine | pmid:27713630 |
| Freag MS et al. | Stealth, biocompatible monoolein-based lyotropic liquid crystalline nanoparticles for enhanced aloe-emodin delivery to breast cancer cells: in vitro and in vivo studies. | 2016 | Int J Nanomedicine | pmid:27703348 |
| Elnaggar YS et al. | Novel piperine-loaded Tween-integrated monoolein cubosomes as brain-targeted oral nanomedicine in Alzheimer's disease: pharmaceutical, biological, and toxicological studies. | 2015 | Int J Nanomedicine | pmid:26346130 |
| Baskaran R et al. | Entrapment of curcumin into monoolein-based liquid crystalline nanoparticle dispersion for enhancement of stability and anticancer activity. | 2014 | Int J Nanomedicine | pmid:25061290 |