| MeSH term | MeSH ID | Detail |
|---|---|---|
| Abscess | D000038 | 13 associated lipids |
| Arthritis | D001168 | 41 associated lipids |
| Body Weight | D001835 | 333 associated lipids |
| Burns | D002056 | 34 associated lipids |
| Edema | D004487 | 152 associated lipids |
| Hypertension, Renovascular | D006978 | 10 associated lipids |
| Psoriasis | D011565 | 47 associated lipids |
| Blood Loss, Surgical | D016063 | 6 associated lipids |
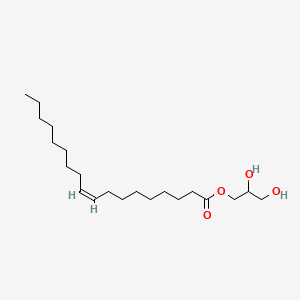
2,3-Dihydroxypropyl oleate
2,3-Dihydroxypropyl oleate is a lipid of Glycerolipids (GL) class. The involved functions are known as enzyme activity and acyltransferase activity. 2,3-dihydroxypropyl oleate often locates in soluble fraction.
Cross Reference
Introduction
To understand associated biological information of 2,3-Dihydroxypropyl oleate, we collected biological information of abnormalities, associated pathways, cellular/molecular locations, biological functions, related genes/proteins, lipids and common seen animal/experimental models with organized paragraphs from literatures.
What diseases are associated with 2,3-Dihydroxypropyl oleate?
There are no associated biomedical information in the current reference collection.
Possible diseases from mapped MeSH terms on references
We collected disease MeSH terms mapped to the references associated with 2,3-Dihydroxypropyl oleate
PubChem Associated disorders and diseases
What pathways are associated with 2,3-Dihydroxypropyl oleate
There are no associated biomedical information in the current reference collection.
PubChem Biomolecular Interactions and Pathways
Link to PubChem Biomolecular Interactions and PathwaysWhat cellular locations are associated with 2,3-Dihydroxypropyl oleate?
Visualization in cellular structure
Associated locations are in red color. Not associated locations are in black.
Related references are published most in these journals:
| Location | Cross reference | Weighted score | Related literatures |
|---|
What functions are associated with 2,3-Dihydroxypropyl oleate?
Related references are published most in these journals:
| Function | Cross reference | Weighted score | Related literatures |
|---|
What lipids are associated with 2,3-Dihydroxypropyl oleate?
There are no associated biomedical information in the current reference collection.
What genes are associated with 2,3-Dihydroxypropyl oleate?
There are no associated biomedical information in the current reference collection.
What common seen animal models are associated with 2,3-Dihydroxypropyl oleate?
There are no associated biomedical information in the current reference collection.
NCBI Entrez Crosslinks
All references with 2,3-Dihydroxypropyl oleate
Download all related citations| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Shah MH and Paradkar A | Effect of HLB of additives on the properties and drug release from the glyceryl monooleate matrices. | 2007 | Eur J Pharm Biopharm | pmid:17353118 |
| Nielsen LH et al. | Microcontainers as an oral delivery system for spray dried cubosomes containing ovalbumin. | 2017 | Eur J Pharm Biopharm | pmid:27993733 |
| Lopes LB et al. | Topical delivery of cyclosporin A: an in vitro study using monoolein as a penetration enhancer. | 2005 | Eur J Pharm Biopharm | pmid:15848052 |
| Steluti R et al. | Topical glycerol monooleate/propylene glycol formulations enhance 5-aminolevulinic acid in vitro skin delivery and in vivo protophorphyrin IX accumulation in hairless mouse skin. | 2005 | Eur J Pharm Biopharm | pmid:15996585 |
| Ahmed AR et al. | Reduction in burst release of PLGA microparticles by incorporation into cubic phase-forming systems. | 2008 | Eur J Pharm Biopharm | pmid:18692569 |
| Onoue S et al. | Inhalable dry-emulsion formulation of cyclosporine A with improved anti-inflammatory effects in experimental asthma/COPD-model rats. | 2012 | Eur J Pharm Biopharm | pmid:22008148 |
| Bittner B et al. | The use of election paramagnetic resonance spectroscopy in early preformulation experiments: the impact of different experimental formulations on the release of a lipophilic spin probe into gastric juice. | 2001 | Eur J Pharm Biopharm | pmid:11226824 |
| Morsi NM et al. | Silver sulfadiazine based cubosome hydrogels for topical treatment of burns: development and in vitro/in vivo characterization. | 2014 | Eur J Pharm Biopharm | pmid:23688805 |
| Sallam AS et al. | Formulation of an oral dosage form utilizing the properties of cubic liquid crystalline phases of glyceryl monooleate. | 2002 | Eur J Pharm Biopharm | pmid:11976023 |
| Esposito E et al. | Effect of nanostructured lipid vehicles on percutaneous absorption of curcumin. | 2014 | Eur J Pharm Biopharm | pmid:24361485 |
| Tiossi RF et al. | In vitro and in vivo evaluation of the delivery of topical formulations containing glycoalkaloids of Solanum lycocarpum fruits. | 2014 | Eur J Pharm Biopharm | pmid:24509413 |
| Rizwan SB et al. | Preparation of phytantriol cubosomes by solvent precursor dilution for the delivery of protein vaccines. | 2011 | Eur J Pharm Biopharm | pmid:21237267 |
| Donida B et al. | Monoolein-based nanoparticles for drug delivery to the central nervous system: A platform for lysosomal storage disorder treatment. | 2018 | Eur J Pharm Biopharm | pmid:30315863 |
| Vithani K et al. | Solubilisation behaviour of poorly water-soluble drugs during digestion of solid SMEDDS. | 2018 | Eur J Pharm Biopharm | pmid:29981444 |
| Simonetti LD et al. | Assessment of the percutaneous penetration of cisplatin: the effect of monoolein and the drug skin penetration pathway. | 2009 | Eur J Pharm Biopharm | pmid:19442727 |
| Zhu Q et al. | A two-stage enzymatic process for synthesis of extremely pure high oleic glycerol monooleate. | 2011 | Enzyme Microb. Technol. | pmid:22112823 |
| Zawalich WS and Zawalich KC | Forskolin-induced desensitization of pancreatic beta-cell insulin secretory responsiveness: possible involvement of impaired information flow in the inositol-lipid cycle. | 1990 | Endocrinology | pmid:1691696 |
| Zawalich WS et al. | Interleukin-1 alpha exerts glucose-dependent stimulatory and inhibitory effects on islet cell phosphoinositide hydrolysis and insulin secretion. | 1989 | Endocrinology | pmid:2539976 |
| Zawalich WS | Phosphoinositide hydrolysis and insulin secretion in response to glucose stimulation are impaired in isolated rat islets by prolonged exposure to the sulfonylurea tolbutamide. | 1989 | Endocrinology | pmid:2544404 |
| Sanandaji N et al. | Comparison of oligonucleotide migration in a bicontinuous cubic phase of monoolein and water and in a fibrous agarose hydrogel. | 2006 | Electrophoresis | pmid:16807936 |
| Lopes I et al. | Toxicity and genotoxicity of organic and inorganic nanoparticles to the bacteria Vibrio fischeri and Salmonella typhimurium. | 2012 | Ecotoxicology | pmid:22314390 |
| Costa-Balogh FO et al. | Drug release from lipid liquid crystalline phases: relation with phase behavior. | 2010 | Drug Dev Ind Pharm | pmid:19848561 |
| Kwon TK and Kim JC | Monoolein cubic phase containing acidic proteinoid: pH-dependent release. | 2011 | Drug Dev Ind Pharm | pmid:20528616 |
| Quiñones OG et al. | In vitro and in vivo influence of penetration enhancers in the topical application of celecoxib. | 2014 | Drug Dev Ind Pharm | pmid:23826859 |
| Réeff J et al. | New sustained-release intraarticular gel formulations based on monolein for local treatment of arthritic diseases. | 2013 | Drug Dev Ind Pharm | pmid:23078519 |
| Montenegro L et al. | In vitro evaluation of idebenone-loaded solid lipid nanoparticles for drug delivery to the brain. | 2011 | Drug Dev Ind Pharm | pmid:21204752 |
| Dai J and Kim JC | Photo responsive monoolein cubic phase containing coumarin-Tween 20 conjugates. | 2013 | Drug Dev Ind Pharm | pmid:23902365 |
| Helledi LS and Schubert L | Release kinetics of acyclovir from a suspension of acyclovir incorporated in a cubic phase delivery system. | 2001 | Drug Dev Ind Pharm | pmid:11794810 |
| Zhou Y et al. | Biological artificial fluid-induced non-lamellar phases in glyceryl monooleate: the kinetics pathway and its digestive process by bile salts. | 2014 | Drug Dev Ind Pharm | pmid:23350691 |
| Ahirrao M and Shrotriya S | In vitro and in vivo evaluation of cubosomal in situ nasal gel containing resveratrol for brain targeting. | 2017 | Drug Dev Ind Pharm | pmid:28574732 |
| Lee J and Kellaway IW | Peptide washout and permeability from glyceryl monooleate buccal delivery systems. | 2002 | Drug Dev Ind Pharm | pmid:12455474 |
| Patil SS et al. | Fabrication of novel GMO/Eudragit E100 nanostructures for enhancing oral bioavailability of carvedilol. | 2016 | Drug Dev Ind Pharm | pmid:26651381 |
| Ganem-Quintanar A et al. | Monoolein: a review of the pharmaceutical applications. | 2000 | Drug Dev Ind Pharm | pmid:10900537 |
| Puri V and Bansal AK | In vitro-in vivo characterization of release modifying agents for parenteral sustained-release ketorolac formulation. | 2004 | Drug Dev Ind Pharm | pmid:15285335 |
| Okonogi S et al. | Development of local injectable dental gel: the influence of certain additives on physicochemical properties of glycerylmonooleate-based formulations. | 2004 | Drug Dev Ind Pharm | pmid:15132177 |
| Rachmawati H et al. | Curcumin nanoemulsion for transdermal application: formulation and evaluation. | 2015 | Drug Dev Ind Pharm | pmid:24502271 |
| Shan-Bin G et al. | Long-term sustained-released in situ gels of a water-insoluble drug amphotericin B for mycotic arthritis intra-articular administration: preparation, in vitro and in vivo evaluation. | 2015 | Drug Dev Ind Pharm | pmid:24502270 |
| Badie H and Abbas H | Novel small self-assembled resveratrol-bearing cubosomes and hexosomes: preparation, charachterization, and ex vivo permeation. | 2018 | Drug Dev Ind Pharm | pmid:30095009 |
| Eskinazi-Budge A et al. | Preparation of emulsifying wax/glyceryl monooleate nanoparticles and evaluation as a delivery system for repurposing simvastatin in bone regeneration. | 2018 | Drug Dev Ind Pharm | pmid:29847182 |
| Peng X et al. | Characterization of cubosomes as a targeted and sustained transdermal delivery system for capsaicin. | 2015 | Drug Des Devel Ther | pmid:26345516 |
| Pham AC et al. | Examining the gastrointestinal transit of lipid-based liquid crystalline systems using whole-animal imaging. | 2015 | Drug Deliv Transl Res | pmid:26328930 |
| Yu X et al. | Melanoma therapy with transdermal mitoxantrone cubic phases. | 2016 | Drug Deliv | pmid:25835224 |
| Aboud HM et al. | Novel in situ gelling vaginal sponges of sildenafil citrate-based cubosomes for uterine targeting. | 2018 | Drug Deliv | pmid:29869515 |
| Zawalich WS et al. | The effect of monooleoylglycerol on insulin secretion from isolated perifused rat islets. | 1989 | Diabetologia | pmid:2668083 |
| Timmers KI et al. | Multiple alterations in insulin responses to glucose in islets from 48-h glucose-infused nondiabetic rats. | 1990 | Diabetes | pmid:1977650 |
| Bhatt AB et al. | Silica nanoparticle stabilization of liquid crystalline lipid dispersions: impact on enzymatic digestion and drug solubilization. | 2015 | Curr Drug Deliv | pmid:25176029 |
| Maurya L et al. | Vitamin E TPGS Emulsified Vinorelbine Bitartrate Loaded Solid Lipid Nanoparticles (SLN): Formulation Development, Optimization and In vitro Characterization. | 2018 | Curr Drug Deliv | pmid:29629662 |
| Komnick H et al. | Is monoacylglycerol as an intermediate of triacylglycerol digestion absorbed by Aeshna cyanea larvae? | 1996 | Comp. Biochem. Physiol. B, Biochem. Mol. Biol. | pmid:8761172 |
| Garti N et al. | Lipolysis and structure controlled drug release from reversed hexagonal mesophase. | 2012 | Colloids Surf B Biointerfaces | pmid:22341515 |
| Ericsson EM et al. | Glycerol monooleate-blood interactions. | 2009 | Colloids Surf B Biointerfaces | pmid:18996684 |