| MeSH term | MeSH ID | Detail |
|---|---|---|
| Burns | D002056 | 34 associated lipids |
| Body Weight | D001835 | 333 associated lipids |
| Edema | D004487 | 152 associated lipids |
| Arthritis | D001168 | 41 associated lipids |
| Abscess | D000038 | 13 associated lipids |
| Psoriasis | D011565 | 47 associated lipids |
| Hypertension, Renovascular | D006978 | 10 associated lipids |
| Blood Loss, Surgical | D016063 | 6 associated lipids |
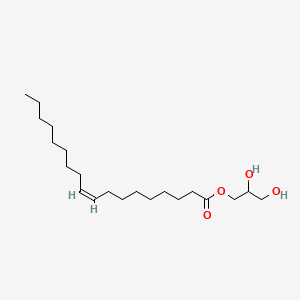
2,3-Dihydroxypropyl oleate
2,3-Dihydroxypropyl oleate is a lipid of Glycerolipids (GL) class. The involved functions are known as enzyme activity and acyltransferase activity. 2,3-dihydroxypropyl oleate often locates in soluble fraction.
Cross Reference
Introduction
To understand associated biological information of 2,3-Dihydroxypropyl oleate, we collected biological information of abnormalities, associated pathways, cellular/molecular locations, biological functions, related genes/proteins, lipids and common seen animal/experimental models with organized paragraphs from literatures.
What diseases are associated with 2,3-Dihydroxypropyl oleate?
There are no associated biomedical information in the current reference collection.
Possible diseases from mapped MeSH terms on references
We collected disease MeSH terms mapped to the references associated with 2,3-Dihydroxypropyl oleate
PubChem Associated disorders and diseases
What pathways are associated with 2,3-Dihydroxypropyl oleate
There are no associated biomedical information in the current reference collection.
PubChem Biomolecular Interactions and Pathways
Link to PubChem Biomolecular Interactions and PathwaysWhat cellular locations are associated with 2,3-Dihydroxypropyl oleate?
Visualization in cellular structure
Associated locations are in red color. Not associated locations are in black.
Related references are published most in these journals:
| Location | Cross reference | Weighted score | Related literatures |
|---|
What functions are associated with 2,3-Dihydroxypropyl oleate?
Related references are published most in these journals:
| Function | Cross reference | Weighted score | Related literatures |
|---|
What lipids are associated with 2,3-Dihydroxypropyl oleate?
There are no associated biomedical information in the current reference collection.
What genes are associated with 2,3-Dihydroxypropyl oleate?
There are no associated biomedical information in the current reference collection.
What common seen animal models are associated with 2,3-Dihydroxypropyl oleate?
There are no associated biomedical information in the current reference collection.
NCBI Entrez Crosslinks
All references with 2,3-Dihydroxypropyl oleate
Download all related citations| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Gan L et al. | Self-assembled liquid crystalline nanoparticles as a novel ophthalmic delivery system for dexamethasone: Improving preocular retention and ocular bioavailability. | 2010 | Int J Pharm | pmid:20558263 |
| Phan S et al. | Evaluating the link between self-assembled mesophase structure and drug release. | 2011 | Int J Pharm | pmid:21963475 |
| Shah MH and Paradkar A | Cubic liquid crystalline glyceryl monooleate matrices for oral delivery of enzyme. | 2005 | Int J Pharm | pmid:15814241 |
| Herai H et al. | Doxorubicin skin penetration from monoolein-containing propylene glycol formulations. | 2007 | Int J Pharm | pmid:17027205 |
| Wörle G et al. | Influence of composition and preparation parameters on the properties of aqueous monoolein dispersions. | 2007 | Int J Pharm | pmid:16987623 |
| Boyd BJ et al. | Lyotropic liquid crystalline phases formed from glycerate surfactants as sustained release drug delivery systems. | 2006 | Int J Pharm | pmid:16413980 |
| Pepe D et al. | Decylglucoside-based microemulsions for cutaneous localization of lycopene and ascorbic acid. | 2012 | Int J Pharm | pmid:22692080 |
| Réeff J et al. | Characterization and optimization of GMO-based gels with long term release for intraarticular administration. | 2013 | Int J Pharm | pmid:23651644 |
| Dash AK et al. | Development of a rectal nicotine delivery system for the treatment of ulcerative colitis. | 1999 | Int J Pharm | pmid:10528093 |
| Siekmann B et al. | Preparation and structural investigations of colloidal dispersions prepared from cubic monoglyceride-water phases. | 2002 | Int J Pharm | pmid:12204563 |
| Gonçalves VSS et al. | Application of RPMI 2650 as a cell model to evaluate solid formulations for intranasal delivery of drugs. | 2016 | Int J Pharm | pmid:27702697 |
| Shi C et al. | Exploring the effect of hydrophilic and hydrophobic structure of grafted polymeric micelles on drug loading. | 2016 | Int J Pharm | pmid:27576669 |
| Sadhale Y and Shah JC | Stabilization of insulin against agitation-induced aggregation by the GMO cubic phase gel. | 1999 | Int J Pharm | pmid:10556740 |
| Sadhale Y and Shah JC | Biological activity of insulin in GMO gels and the effect of agitation. | 1999 | Int J Pharm | pmid:10556741 |
| Mohamed AI et al. | In-vivo evaluation of clindamycin release from glyceryl monooleate-alginate microspheres by NIR spectroscopy. | 2015 | Int J Pharm | pmid:26276253 |
| Luo Q et al. | A novel glyceryl monoolein-bearing cubosomes for gambogenic acid: Preparation, cytotoxicity and intracellular uptake. | 2015 | Int J Pharm | pmid:26209071 |
| Réeff J et al. | Development and evaluation in vitro and in vivo of injectable hydrolipidic gels with sustained-release properties for the management of articular pathologies such as osteoarthritis. | 2015 | Int J Pharm | pmid:25934426 |
| Sintov AC | Transdermal delivery of curcumin via microemulsion. | 2015 | Int J Pharm | pmid:25655717 |
| Zhang Y et al. | Transdermal baicalin delivery using diethylene glycol monoethyl ether-mediated cubic phase gel. | 2015 | Int J Pharm | pmid:25543112 |
| Milak S and Zimmer A | Glycerol monooleate liquid crystalline phases used in drug delivery systems. | 2015 | Int J Pharm | pmid:25479099 |
| Du LR et al. | Development and evaluation of liquid embolic agents based on liquid crystalline material of glyceryl monooleate. | 2014 | Int J Pharm | pmid:24858389 |
| Liu Y et al. | Preparation and evaluation of glyceryl monooleate-coated hollow-bioadhesive microspheres for gastroretentive drug delivery. | 2011 | Int J Pharm | pmid:21540088 |
| Lee KW et al. | Nanostructure of liquid crystalline matrix determines in vitro sustained release and in vivo oral absorption kinetics for hydrophilic model drugs. | 2009 | Int J Pharm | pmid:18790030 |
| Libster D et al. | Molecular interactions in reverse hexagonal mesophase in the presence of Cyclosporin A. | 2009 | Int J Pharm | pmid:18977286 |
| Valente F et al. | Evaluation of toxicity of glycerol monooleate nanoparticles on PC12 cell line. | 2018 | Int J Pharm | pmid:29366940 |
| Isaksson S et al. | Influence of three alloplastic materials on calvarial bone healing. An experimental evaluation of HTR-polymer, lactomer beads, and a carrier gel. | 1993 | Int J Oral Maxillofac Surg | pmid:8106817 |
| Shen J et al. | Preparation and evaluation of a self-nanoemulsifying drug delivery system loaded with Akebia saponin D-phospholipid complex. | 2016 | Int J Nanomedicine | pmid:27713630 |
| Freag MS et al. | Stealth, biocompatible monoolein-based lyotropic liquid crystalline nanoparticles for enhanced aloe-emodin delivery to breast cancer cells: in vitro and in vivo studies. | 2016 | Int J Nanomedicine | pmid:27703348 |
| Elnaggar YS et al. | Novel piperine-loaded Tween-integrated monoolein cubosomes as brain-targeted oral nanomedicine in Alzheimer's disease: pharmaceutical, biological, and toxicological studies. | 2015 | Int J Nanomedicine | pmid:26346130 |
| Baskaran R et al. | Entrapment of curcumin into monoolein-based liquid crystalline nanoparticle dispersion for enhancement of stability and anticancer activity. | 2014 | Int J Nanomedicine | pmid:25061290 |
| Issa JP et al. | Biological evaluation of the bone healing process after application of two potentially osteogenic proteins: an animal experimental model. | 2012 | Gerodontology | pmid:22970792 |
| Pomès R and Yu CH | Relay and blockage of protons in water chains. | 2003 | Front. Biosci. | pmid:12957853 |
| Longnecker DS et al. | Pancreatic carcinogenesis in azaserine-treated rats: inhibition by a solvent mixture in the diet. | 1987 | Food Chem. Toxicol. | pmid:3500902 |
| Deleers M and Malaisse WJ | Facilitated fusion of liposomes with glycerol monoleate planar bilayer. | 1981 | FEBS Lett. | pmid:7297692 |
| Crawford GE and Earnshaw JC | Photon correlation spectroscopy as a probe of planar lipid bilayer phase transitions. | 1984 | Eur. Biophys. J. | pmid:6468342 |
| Kolev V et al. | Unit cell structure of water-filled monoolein in inverted hexagonal mesophase in the presence of incorporated tricaprylin and entrapped lysozyme. | 2016 | Eur. Biophys. J. | pmid:26424533 |
| Leroy S et al. | Anisotropic surface melting in lyotropic cubic crystals: part 2: facet-by-facet melting at Ia3d/vapor interfaces. | 2006 | Eur Phys J E Soft Matter | pmid:16733635 |
| Lopes LB et al. | Enhancement of skin penetration of vitamin K using monoolein-based liquid crystalline systems. | 2007 | Eur J Pharm Sci | pmid:17900879 |
| Rossetti FC et al. | Optimization of protoporphyrin IX skin delivery for topical photodynamic therapy: Nanodispersions of liquid-crystalline phase as nanocarriers. | 2016 | Eur J Pharm Sci | pmid:26657201 |
| Wörle G et al. | Transformation of vesicular into cubic nanoparticles by autoclaving of aqueous monoolein/poloxamer dispersions. | 2006 | Eur J Pharm Sci | pmid:16157479 |
| Nielsen LS et al. | Bioadhesive drug delivery systems. I. Characterisation of mucoadhesive properties of systems based on glyceryl mono-oleate and glyceryl monolinoleate. | 1998 | Eur J Pharm Sci | pmid:9795071 |
| Engström S et al. | Cubic phases for studies of drug partition into lipid bilayers. | 1999 | Eur J Pharm Sci | pmid:10425374 |
| Borgheti-Cardoso LN et al. | An in situ gelling liquid crystalline system based on monoglycerides and polyethylenimine for local delivery of siRNAs. | 2015 | Eur J Pharm Sci | pmid:25917525 |
| Patil SS et al. | Liquid crystalline phase as a probe for crystal engineering of lactose: carrier for pulmonary drug delivery. | 2015 | Eur J Pharm Sci | pmid:25460546 |
| Lopes LB et al. | Topical delivery of cyclosporin A: an in vitro study using monoolein as a penetration enhancer. | 2005 | Eur J Pharm Biopharm | pmid:15848052 |
| Steluti R et al. | Topical glycerol monooleate/propylene glycol formulations enhance 5-aminolevulinic acid in vitro skin delivery and in vivo protophorphyrin IX accumulation in hairless mouse skin. | 2005 | Eur J Pharm Biopharm | pmid:15996585 |
| Ahmed AR et al. | Reduction in burst release of PLGA microparticles by incorporation into cubic phase-forming systems. | 2008 | Eur J Pharm Biopharm | pmid:18692569 |
| Vicentini FT et al. | Liquid crystalline phase nanodispersions enable skin delivery of siRNA. | 2013 | Eur J Pharm Biopharm | pmid:23010565 |
| Donida B et al. | Monoolein-based nanoparticles for drug delivery to the central nervous system: A platform for lysosomal storage disorder treatment. | 2018 | Eur J Pharm Biopharm | pmid:30315863 |
| Vithani K et al. | Solubilisation behaviour of poorly water-soluble drugs during digestion of solid SMEDDS. | 2018 | Eur J Pharm Biopharm | pmid:29981444 |