| MeSH term | MeSH ID | Detail |
|---|---|---|
| Burns | D002056 | 34 associated lipids |
| Body Weight | D001835 | 333 associated lipids |
| Edema | D004487 | 152 associated lipids |
| Arthritis | D001168 | 41 associated lipids |
| Abscess | D000038 | 13 associated lipids |
| Psoriasis | D011565 | 47 associated lipids |
| Hypertension, Renovascular | D006978 | 10 associated lipids |
| Blood Loss, Surgical | D016063 | 6 associated lipids |
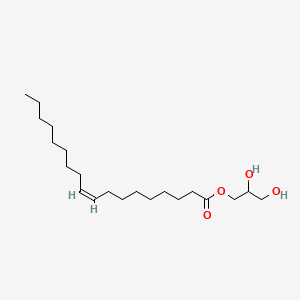
2,3-Dihydroxypropyl oleate
2,3-Dihydroxypropyl oleate is a lipid of Glycerolipids (GL) class. The involved functions are known as enzyme activity and acyltransferase activity. 2,3-dihydroxypropyl oleate often locates in soluble fraction.
Cross Reference
Introduction
To understand associated biological information of 2,3-Dihydroxypropyl oleate, we collected biological information of abnormalities, associated pathways, cellular/molecular locations, biological functions, related genes/proteins, lipids and common seen animal/experimental models with organized paragraphs from literatures.
What diseases are associated with 2,3-Dihydroxypropyl oleate?
There are no associated biomedical information in the current reference collection.
Possible diseases from mapped MeSH terms on references
We collected disease MeSH terms mapped to the references associated with 2,3-Dihydroxypropyl oleate
PubChem Associated disorders and diseases
What pathways are associated with 2,3-Dihydroxypropyl oleate
There are no associated biomedical information in the current reference collection.
PubChem Biomolecular Interactions and Pathways
Link to PubChem Biomolecular Interactions and PathwaysWhat cellular locations are associated with 2,3-Dihydroxypropyl oleate?
Visualization in cellular structure
Associated locations are in red color. Not associated locations are in black.
Related references are published most in these journals:
| Location | Cross reference | Weighted score | Related literatures |
|---|
What functions are associated with 2,3-Dihydroxypropyl oleate?
Related references are published most in these journals:
| Function | Cross reference | Weighted score | Related literatures |
|---|
What lipids are associated with 2,3-Dihydroxypropyl oleate?
There are no associated biomedical information in the current reference collection.
What genes are associated with 2,3-Dihydroxypropyl oleate?
There are no associated biomedical information in the current reference collection.
What common seen animal models are associated with 2,3-Dihydroxypropyl oleate?
There are no associated biomedical information in the current reference collection.
NCBI Entrez Crosslinks
All references with 2,3-Dihydroxypropyl oleate
Download all related citations| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Peng X et al. | Characterization of cubosomes as a targeted and sustained transdermal delivery system for capsaicin. | 2015 | Drug Des Devel Ther | pmid:26345516 |
| Costa-Balogh FO et al. | Drug release from lipid liquid crystalline phases: relation with phase behavior. | 2010 | Drug Dev Ind Pharm | pmid:19848561 |
| Kwon TK and Kim JC | Monoolein cubic phase containing acidic proteinoid: pH-dependent release. | 2011 | Drug Dev Ind Pharm | pmid:20528616 |
| Quiñones OG et al. | In vitro and in vivo influence of penetration enhancers in the topical application of celecoxib. | 2014 | Drug Dev Ind Pharm | pmid:23826859 |
| Réeff J et al. | New sustained-release intraarticular gel formulations based on monolein for local treatment of arthritic diseases. | 2013 | Drug Dev Ind Pharm | pmid:23078519 |
| Montenegro L et al. | In vitro evaluation of idebenone-loaded solid lipid nanoparticles for drug delivery to the brain. | 2011 | Drug Dev Ind Pharm | pmid:21204752 |
| Dai J and Kim JC | Photo responsive monoolein cubic phase containing coumarin-Tween 20 conjugates. | 2013 | Drug Dev Ind Pharm | pmid:23902365 |
| Helledi LS and Schubert L | Release kinetics of acyclovir from a suspension of acyclovir incorporated in a cubic phase delivery system. | 2001 | Drug Dev Ind Pharm | pmid:11794810 |
| Zhou Y et al. | Biological artificial fluid-induced non-lamellar phases in glyceryl monooleate: the kinetics pathway and its digestive process by bile salts. | 2014 | Drug Dev Ind Pharm | pmid:23350691 |
| Ahirrao M and Shrotriya S | In vitro and in vivo evaluation of cubosomal in situ nasal gel containing resveratrol for brain targeting. | 2017 | Drug Dev Ind Pharm | pmid:28574732 |
| Lee J and Kellaway IW | Peptide washout and permeability from glyceryl monooleate buccal delivery systems. | 2002 | Drug Dev Ind Pharm | pmid:12455474 |
| Patil SS et al. | Fabrication of novel GMO/Eudragit E100 nanostructures for enhancing oral bioavailability of carvedilol. | 2016 | Drug Dev Ind Pharm | pmid:26651381 |
| Ganem-Quintanar A et al. | Monoolein: a review of the pharmaceutical applications. | 2000 | Drug Dev Ind Pharm | pmid:10900537 |
| Puri V and Bansal AK | In vitro-in vivo characterization of release modifying agents for parenteral sustained-release ketorolac formulation. | 2004 | Drug Dev Ind Pharm | pmid:15285335 |
| Okonogi S et al. | Development of local injectable dental gel: the influence of certain additives on physicochemical properties of glycerylmonooleate-based formulations. | 2004 | Drug Dev Ind Pharm | pmid:15132177 |
| Rachmawati H et al. | Curcumin nanoemulsion for transdermal application: formulation and evaluation. | 2015 | Drug Dev Ind Pharm | pmid:24502271 |
| Shan-Bin G et al. | Long-term sustained-released in situ gels of a water-insoluble drug amphotericin B for mycotic arthritis intra-articular administration: preparation, in vitro and in vivo evaluation. | 2015 | Drug Dev Ind Pharm | pmid:24502270 |
| Badie H and Abbas H | Novel small self-assembled resveratrol-bearing cubosomes and hexosomes: preparation, charachterization, and ex vivo permeation. | 2018 | Drug Dev Ind Pharm | pmid:30095009 |
| Eskinazi-Budge A et al. | Preparation of emulsifying wax/glyceryl monooleate nanoparticles and evaluation as a delivery system for repurposing simvastatin in bone regeneration. | 2018 | Drug Dev Ind Pharm | pmid:29847182 |
| Lopes I et al. | Toxicity and genotoxicity of organic and inorganic nanoparticles to the bacteria Vibrio fischeri and Salmonella typhimurium. | 2012 | Ecotoxicology | pmid:22314390 |