| MeSH term | MeSH ID | Detail |
|---|---|---|
| Burns | D002056 | 34 associated lipids |
| Body Weight | D001835 | 333 associated lipids |
| Edema | D004487 | 152 associated lipids |
| Arthritis | D001168 | 41 associated lipids |
| Abscess | D000038 | 13 associated lipids |
| Psoriasis | D011565 | 47 associated lipids |
| Hypertension, Renovascular | D006978 | 10 associated lipids |
| Blood Loss, Surgical | D016063 | 6 associated lipids |
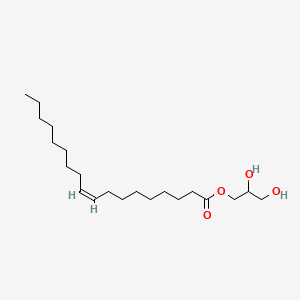
2,3-Dihydroxypropyl oleate
2,3-Dihydroxypropyl oleate is a lipid of Glycerolipids (GL) class. The involved functions are known as enzyme activity and acyltransferase activity. 2,3-dihydroxypropyl oleate often locates in soluble fraction.
Cross Reference
Introduction
To understand associated biological information of 2,3-Dihydroxypropyl oleate, we collected biological information of abnormalities, associated pathways, cellular/molecular locations, biological functions, related genes/proteins, lipids and common seen animal/experimental models with organized paragraphs from literatures.
What diseases are associated with 2,3-Dihydroxypropyl oleate?
There are no associated biomedical information in the current reference collection.
Possible diseases from mapped MeSH terms on references
We collected disease MeSH terms mapped to the references associated with 2,3-Dihydroxypropyl oleate
PubChem Associated disorders and diseases
What pathways are associated with 2,3-Dihydroxypropyl oleate
There are no associated biomedical information in the current reference collection.
PubChem Biomolecular Interactions and Pathways
Link to PubChem Biomolecular Interactions and PathwaysWhat cellular locations are associated with 2,3-Dihydroxypropyl oleate?
Visualization in cellular structure
Associated locations are in red color. Not associated locations are in black.
Related references are published most in these journals:
| Location | Cross reference | Weighted score | Related literatures |
|---|
What functions are associated with 2,3-Dihydroxypropyl oleate?
Related references are published most in these journals:
| Function | Cross reference | Weighted score | Related literatures |
|---|
What lipids are associated with 2,3-Dihydroxypropyl oleate?
There are no associated biomedical information in the current reference collection.
What genes are associated with 2,3-Dihydroxypropyl oleate?
There are no associated biomedical information in the current reference collection.
What common seen animal models are associated with 2,3-Dihydroxypropyl oleate?
There are no associated biomedical information in the current reference collection.
NCBI Entrez Crosslinks
All references with 2,3-Dihydroxypropyl oleate
Download all related citations| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Abe S and Takahashi H | A comparative study of the effects of dimethylsulfoxide and glycerol on the bicontinuous cubic structure of hydrated monoolein and its phase behavior. | 2007 | Chem. Phys. Lipids | pmid:17451662 |
| Engblom J | Bicontinuous cubic phase: a model for investigating the effects on a lipid bilayer due to a foreign substance. | 1996 | Chem. Phys. Lipids | pmid:9022220 |
| Clogston J et al. | Phase behavior of a monoacylglycerol: (myverol 18-99K)/water system. | 2000 | Chem. Phys. Lipids | pmid:11090848 |
| Barauskas J et al. | Solubilization of ubiquinone-10 in the lamellar and bicontinuous cubic phases of aqueous monoolein. | 1999 | Chem. Phys. Lipids | pmid:10192931 |
| Barauskas J et al. | Towards redox active liquid crystalline phases of lipids: a monoolein/water system with entrapped derivatives of ferrocene. | 2003 | Chem. Phys. Lipids | pmid:12637167 |
| Rosa A et al. | Monoolein-based cubosomes affect lipid profile in HeLa cells. | 2015 | Chem. Phys. Lipids | pmid:26341749 |
| Lee SJ et al. | Bioadhesive drug delivery system using glyceryl monooleate for the intravesical administration of paclitaxel. | 2005 | Chemotherapy | pmid:16224181 |
| Issa JP et al. | Effect of recombinant human bone morphogenetic protein-2 on bone formation in the acute distraction osteogenesis of rat mandibles. | 2009 | Clin Oral Implants Res | pmid:19719732 |
| Nilsson-Ehle P | On the specificity of assays for lipases in postheparin plasma. | 1984 | Clin. Chim. Acta | pmid:6488561 |
| Yariv D et al. | In vitro permeation of diclofenac salts from lyotropic liquid crystalline systems. | 2010 | Colloids Surf B Biointerfaces | pmid:20363601 |
| Cohen-Avrahami M et al. | H(II) mesophase and peptide cell-penetrating enhancers for improved transdermal delivery of sodium diclofenac. | 2010 | Colloids Surf B Biointerfaces | pmid:20189781 |
| Amar-Yuli I and Garti N | Transitions induced by solubilized fat into reverse hexagonal mesophases. | 2005 | Colloids Surf B Biointerfaces | pmid:15921902 |
| Imberg A | On the microstructures of ethyl acetate/monoolein/water. | 2006 | Colloids Surf B Biointerfaces | pmid:17084597 |
| Garti N et al. | Lipolysis and structure controlled drug release from reversed hexagonal mesophase. | 2012 | Colloids Surf B Biointerfaces | pmid:22341515 |
| Hirano S et al. | Continuous monitoring of phospholipid vesicle hydrolysis by phospholipase D (PLD) reveals differences in hydrolysis by PLDs from 2 Streptomyces species. | 2012 | Colloids Surf B Biointerfaces | pmid:22391322 |
| Bitan-Cherbakovsky L et al. | Structural characterization of lyotropic liquid crystals containing a dendrimer for solubilization and release of gallic acid. | 2013 | Colloids Surf B Biointerfaces | pmid:23973908 |
| Murgia S et al. | Cubosome formulations stabilized by a dansyl-conjugated block copolymer for possible nanomedicine applications. | 2015 | Colloids Surf B Biointerfaces | pmid:25829131 |
| Kim JC et al. | Monoolein cubic phases containing hydrogen peroxide. | 2004 | Colloids Surf B Biointerfaces | pmid:15276632 |
| Leesajakul W et al. | Interaction of cubosomes with plasma components resulting in the destabilization of cubosomes in plasma. | 2004 | Colloids Surf B Biointerfaces | pmid:15261065 |
| Esposito E et al. | Monoolein liquid crystalline phases for topical delivery of crocetin. | 2018 | Colloids Surf B Biointerfaces | pmid:30015140 |
| Ericsson EM et al. | Glycerol monooleate-blood interactions. | 2009 | Colloids Surf B Biointerfaces | pmid:18996684 |
| Mishraki T et al. | Lysozyme entrapped within reverse hexagonal mesophases: physical properties and structural behavior. | 2010 | Colloids Surf B Biointerfaces | pmid:19748240 |
| Komnick H et al. | Is monoacylglycerol as an intermediate of triacylglycerol digestion absorbed by Aeshna cyanea larvae? | 1996 | Comp. Biochem. Physiol. B, Biochem. Mol. Biol. | pmid:8761172 |
| Bhatt AB et al. | Silica nanoparticle stabilization of liquid crystalline lipid dispersions: impact on enzymatic digestion and drug solubilization. | 2015 | Curr Drug Deliv | pmid:25176029 |
| Maurya L et al. | Vitamin E TPGS Emulsified Vinorelbine Bitartrate Loaded Solid Lipid Nanoparticles (SLN): Formulation Development, Optimization and In vitro Characterization. | 2018 | Curr Drug Deliv | pmid:29629662 |
| Timmers KI et al. | Multiple alterations in insulin responses to glucose in islets from 48-h glucose-infused nondiabetic rats. | 1990 | Diabetes | pmid:1977650 |
| Zawalich WS et al. | The effect of monooleoylglycerol on insulin secretion from isolated perifused rat islets. | 1989 | Diabetologia | pmid:2668083 |
| Yu X et al. | Melanoma therapy with transdermal mitoxantrone cubic phases. | 2016 | Drug Deliv | pmid:25835224 |
| Aboud HM et al. | Novel in situ gelling vaginal sponges of sildenafil citrate-based cubosomes for uterine targeting. | 2018 | Drug Deliv | pmid:29869515 |
| Pham AC et al. | Examining the gastrointestinal transit of lipid-based liquid crystalline systems using whole-animal imaging. | 2015 | Drug Deliv Transl Res | pmid:26328930 |
| Peng X et al. | Characterization of cubosomes as a targeted and sustained transdermal delivery system for capsaicin. | 2015 | Drug Des Devel Ther | pmid:26345516 |
| Costa-Balogh FO et al. | Drug release from lipid liquid crystalline phases: relation with phase behavior. | 2010 | Drug Dev Ind Pharm | pmid:19848561 |
| Kwon TK and Kim JC | Monoolein cubic phase containing acidic proteinoid: pH-dependent release. | 2011 | Drug Dev Ind Pharm | pmid:20528616 |
| Quiñones OG et al. | In vitro and in vivo influence of penetration enhancers in the topical application of celecoxib. | 2014 | Drug Dev Ind Pharm | pmid:23826859 |
| Réeff J et al. | New sustained-release intraarticular gel formulations based on monolein for local treatment of arthritic diseases. | 2013 | Drug Dev Ind Pharm | pmid:23078519 |
| Montenegro L et al. | In vitro evaluation of idebenone-loaded solid lipid nanoparticles for drug delivery to the brain. | 2011 | Drug Dev Ind Pharm | pmid:21204752 |
| Dai J and Kim JC | Photo responsive monoolein cubic phase containing coumarin-Tween 20 conjugates. | 2013 | Drug Dev Ind Pharm | pmid:23902365 |
| Helledi LS and Schubert L | Release kinetics of acyclovir from a suspension of acyclovir incorporated in a cubic phase delivery system. | 2001 | Drug Dev Ind Pharm | pmid:11794810 |
| Zhou Y et al. | Biological artificial fluid-induced non-lamellar phases in glyceryl monooleate: the kinetics pathway and its digestive process by bile salts. | 2014 | Drug Dev Ind Pharm | pmid:23350691 |
| Ahirrao M and Shrotriya S | In vitro and in vivo evaluation of cubosomal in situ nasal gel containing resveratrol for brain targeting. | 2017 | Drug Dev Ind Pharm | pmid:28574732 |
| Lee J and Kellaway IW | Peptide washout and permeability from glyceryl monooleate buccal delivery systems. | 2002 | Drug Dev Ind Pharm | pmid:12455474 |
| Patil SS et al. | Fabrication of novel GMO/Eudragit E100 nanostructures for enhancing oral bioavailability of carvedilol. | 2016 | Drug Dev Ind Pharm | pmid:26651381 |
| Ganem-Quintanar A et al. | Monoolein: a review of the pharmaceutical applications. | 2000 | Drug Dev Ind Pharm | pmid:10900537 |
| Puri V and Bansal AK | In vitro-in vivo characterization of release modifying agents for parenteral sustained-release ketorolac formulation. | 2004 | Drug Dev Ind Pharm | pmid:15285335 |
| Okonogi S et al. | Development of local injectable dental gel: the influence of certain additives on physicochemical properties of glycerylmonooleate-based formulations. | 2004 | Drug Dev Ind Pharm | pmid:15132177 |
| Rachmawati H et al. | Curcumin nanoemulsion for transdermal application: formulation and evaluation. | 2015 | Drug Dev Ind Pharm | pmid:24502271 |
| Shan-Bin G et al. | Long-term sustained-released in situ gels of a water-insoluble drug amphotericin B for mycotic arthritis intra-articular administration: preparation, in vitro and in vivo evaluation. | 2015 | Drug Dev Ind Pharm | pmid:24502270 |
| Badie H and Abbas H | Novel small self-assembled resveratrol-bearing cubosomes and hexosomes: preparation, charachterization, and ex vivo permeation. | 2018 | Drug Dev Ind Pharm | pmid:30095009 |
| Eskinazi-Budge A et al. | Preparation of emulsifying wax/glyceryl monooleate nanoparticles and evaluation as a delivery system for repurposing simvastatin in bone regeneration. | 2018 | Drug Dev Ind Pharm | pmid:29847182 |
| Lopes I et al. | Toxicity and genotoxicity of organic and inorganic nanoparticles to the bacteria Vibrio fischeri and Salmonella typhimurium. | 2012 | Ecotoxicology | pmid:22314390 |