| MeSH term | MeSH ID | Detail |
|---|---|---|
| Mycobacterium Infections | D009164 | 7 associated lipids |
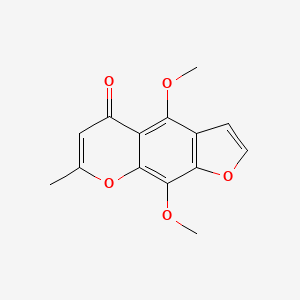
khellin
Khellin is a lipid of Polyketides (PK) class. The involved functions are known as Diastasis and Selection, Genetic.
Cross Reference
Introduction
To understand associated biological information of khellin, we collected biological information of abnormalities, associated pathways, cellular/molecular locations, biological functions, related genes/proteins, lipids and common seen animal/experimental models with organized paragraphs from literatures.
What diseases are associated with khellin?
There are no associated biomedical information in the current reference collection.
Possible diseases from mapped MeSH terms on references
We collected disease MeSH terms mapped to the references associated with khellin
PubChem Associated disorders and diseases
What pathways are associated with khellin
There are no associated biomedical information in the current reference collection.
PubChem Biomolecular Interactions and Pathways
Link to PubChem Biomolecular Interactions and PathwaysWhat cellular locations are associated with khellin?
There are no associated biomedical information in the current reference collection.
What functions are associated with khellin?
Related references are published most in these journals:
| Function | Cross reference | Weighted score | Related literatures |
|---|
What lipids are associated with khellin?
There are no associated biomedical information in the current reference collection.
What genes are associated with khellin?
There are no associated biomedical information in the current reference collection.
What common seen animal models are associated with khellin?
There are no associated biomedical information in the current reference collection.
NCBI Entrez Crosslinks
All references with khellin
Download all related citations| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Janssen S et al. | Exploring prospects of novel drugs for tuberculosis. | 2012 | Drug Des Devel Ther | pmid:22973091 |
| Normoyle KP and Brieher WM | Cyclase-associated protein (CAP) acts directly on F-actin to accelerate cofilin-mediated actin severing across the range of physiological pH. | 2012 | J. Biol. Chem. | pmid:22904322 |
| Koo MS et al. | Strain specific transcriptional response in Mycobacterium tuberculosis infected macrophages. | 2012 | Cell Commun. Signal | pmid:22280836 |
| Günther J et al. | Lipopolysaccharide priming enhances expression of effectors of immune defence while decreasing expression of pro-inflammatory cytokines in mammary epithelia cells from cows. | 2012 | BMC Genomics | pmid:22235868 |
| Morley SC | The actin-bundling protein L-plastin: a critical regulator of immune cell function. | 2012 | Int J Cell Biol | pmid:22194750 |
| Lapalikar GV et al. | F420H2-dependent degradation of aflatoxin and other furanocoumarins is widespread throughout the actinomycetales. | 2012 | PLoS ONE | pmid:22383957 |
| Kilicaslan I and Coskun S | Spontaneous stone passage: is it Ammi visnaga effect? | 2012 | Urol. Res. | pmid:22990409 |
| Haug KG et al. | Pharmacokinetic evaluation of visnagin and Ammi visnaga aqueous extract after oral administration in rats. | 2012 | Planta Med. | pmid:23096256 |
| Hölttä-Vuori M et al. | Endosomal actin remodeling by coronin-1A controls lipoprotein uptake and degradation in macrophages. | 2012 | Circ. Res. | pmid:22223354 |
| Seto S et al. | Coronin-1a inhibits autophagosome formation around Mycobacterium tuberculosis-containing phagosomes and assists mycobacterial survival in macrophages. | 2012 | Cell. Microbiol. | pmid:22256790 |