| MeSH term | MeSH ID | Detail |
|---|---|---|
| Metabolic Syndrome | D024821 | 44 associated lipids |
| Xanthomatosis | D014973 | 17 associated lipids |
| Hypolipoproteinemias | D007009 | 9 associated lipids |
| Hyperlipidemias | D006949 | 73 associated lipids |
| Hyperlipoproteinemia Type II | D006938 | 22 associated lipids |
| Hypercholesterolemia | D006937 | 91 associated lipids |
| Coronary Disease | D003327 | 70 associated lipids |
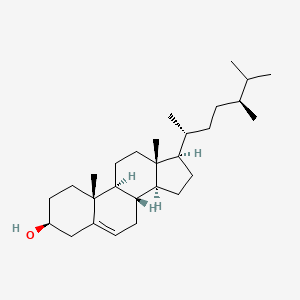
22,23-dihydrobrassicasterol
22,23-dihydrobrassicasterol is a lipid of Sterol Lipids (ST) class. 22,23-dihydrobrassicasterol is associated with abnormalities such as Diabetes, Macular degeneration, Drusen, Systemic disease and Diabetes Mellitus. The involved functions are known as cholesterol metabolism, Synthesis, Intestinal Absorption, Liver function and cholesterol absorption. 22,23-dihydrobrassicasterol often locates in Back and Cell membrane. The associated genes with 22,23-dihydrobrassicasterol are apolipoprotein E-3. The related lipids are Total cholesterol, campesterol, lathosterol, Fatty Acids, Nonesterified and Cholesterol, Dietary.
Cross Reference
Introduction
To understand associated biological information of 22,23-dihydrobrassicasterol, we collected biological information of abnormalities, associated pathways, cellular/molecular locations, biological functions, related genes/proteins, lipids and common seen animal/experimental models with organized paragraphs from literatures.
What diseases are associated with 22,23-dihydrobrassicasterol?
22,23-dihydrobrassicasterol is suspected in Diabetes, Macular degeneration, Drusen, Systemic disease, Diabetes Mellitus, Liver diseases and other diseases in descending order of the highest number of associated sentences.
Related references are mostly published in these journals:
| Disease | Cross reference | Weighted score | Related literature |
|---|
Possible diseases from mapped MeSH terms on references
We collected disease MeSH terms mapped to the references associated with 22,23-dihydrobrassicasterol
PubChem Associated disorders and diseases
What pathways are associated with 22,23-dihydrobrassicasterol
There are no associated biomedical information in the current reference collection.
PubChem Biomolecular Interactions and Pathways
Link to PubChem Biomolecular Interactions and PathwaysWhat cellular locations are associated with 22,23-dihydrobrassicasterol?
Visualization in cellular structure
Associated locations are in red color. Not associated locations are in black.
Related references are published most in these journals:
| Location | Cross reference | Weighted score | Related literatures |
|---|
What functions are associated with 22,23-dihydrobrassicasterol?
Related references are published most in these journals:
| Function | Cross reference | Weighted score | Related literatures |
|---|
What lipids are associated with 22,23-dihydrobrassicasterol?
Related references are published most in these journals:
| Lipid concept | Cross reference | Weighted score | Related literatures |
|---|
What genes are associated with 22,23-dihydrobrassicasterol?
Related references are published most in these journals:
| Gene | Cross reference | Weighted score | Related literatures |
|---|
What common seen animal models are associated with 22,23-dihydrobrassicasterol?
There are no associated biomedical information in the current reference collection.
NCBI Entrez Crosslinks
All references with 22,23-dihydrobrassicasterol
Download all related citations| Authors | Title | Published | Journal | PubMed Link |
|---|---|---|---|---|
| Ramos SC et al. | The role of soluble fiber intake in patients under highly effective lipid-lowering therapy. | 2011 | Nutr J | pmid:21810257 |
| Ramprasath VR et al. | Consumption of a dietary portfolio of cholesterol lowering foods improves blood lipids without affecting concentrations of fat soluble compounds. | 2014 | Nutr J | pmid:25326876 |
| Strom SS et al. | Phytoestrogen intake and prostate cancer: a case-control study using a new database. | 1999 | Nutr Cancer | pmid:10227039 |
| van den Kommer TN et al. | The role of extracerebral cholesterol homeostasis and ApoE e4 in cognitive decline. | 2012 | Neurobiol. Aging | pmid:21482441 |
| Teunissen CE et al. | Serum cholesterol, precursors and metabolites and cognitive performance in an aging population. | 2003 Jan-Feb | Neurobiol. Aging | pmid:12493560 |
| Gerloff T et al. | Influence of the SLCO1B1*1b and *5 haplotypes on pravastatin's cholesterol lowering capabilities and basal sterol serum levels. | 2006 | Naunyn Schmiedebergs Arch. Pharmacol. | pmid:16568260 |
| Sabudak T et al. | Potent tyrosinase inhibitors from Trifolium balansae. | 2006 | Nat. Prod. Res. | pmid:16901809 |
| Arfaoui MO et al. | Variation in oil content, fatty acid and phytosterols profile of Onopordum acanthium L. during seed development. | 2014 | Nat. Prod. Res. | pmid:25103576 |
| de Araújo MF et al. | Simiranes A and B: erythroxylanes diterpenes and other compounds from Simira eliezeriana (Rubiaceae). | 2011 | Nat. Prod. Res. | pmid:21936665 |
| Miettinen TA et al. | Reduction of serum cholesterol with sitostanol-ester margarine in a mildly hypercholesterolemic population. | 1995 | N. Engl. J. Med. | pmid:7566021 |
| Moreno-Anzúrez NE et al. | A Cytotoxic and Anti-inflammatory Campesterol Derivative from Genetically Transformed Hairy Roots of Lopezia racemosa Cav. (Onagraceae). | 2017 | Molecules | pmid:28085103 |
| Salinas R et al. | Production of the anti-inflammatory compound 6-O-palmitoyl-3-O-β-D-glucopyranosylcampesterol by Callus cultures of Lopezia racemosa Cav. (Onagraceae). | 2014 | Molecules | pmid:24962399 |
| Malek SN et al. | Cytotoxic components of Pereskia bleo (Kunth) DC. (Cactaceae) leaves. | 2009 | Molecules | pmid:19471192 |
| Morrison AH and Ritter KS | Effect of host insect sterols on the development and sterol composition of Steinernema feltiae. | 1986 | Mol. Biochem. Parasitol. | pmid:3724794 |
| Labonté MÈ et al. | Comparison of the impact of trans fatty acids from ruminant and industrial sources on surrogate markers of cholesterol homeostasis in healthy men. | 2011 | Mol Nutr Food Res | pmid:21656668 |
| Aherne SA and O'Brien NM | Modulation of cytokine production by plant sterols in stimulated human Jurkat T cells. | 2008 | Mol Nutr Food Res | pmid:18465778 |
| Plat J et al. | Preferential campesterol incorporation into various tissues in apolipoprotein E*3-Leiden mice consuming plant sterols or stanols. | 2008 | Metab. Clin. Exp. | pmid:18702950 |
| Kempen HJ et al. | Lathosterol level in plasma is elevated in type III hyperlipoproteinemia, but not in non-type III subjects with apolipoprotein E2/2 phenotype, nor in type IIa or IIb hyperlipoproteinemia. | 1991 | Metab. Clin. Exp. | pmid:2000034 |
| Sudhop T et al. | Serum plant sterols as a potential risk factor for coronary heart disease. | 2002 | Metab. Clin. Exp. | pmid:12489060 |
| Ikeda I et al. | Mechanisms of phytosterolemia in stroke-prone spontaneously hypertensive and WKY rats. | 2001 | Metab. Clin. Exp. | pmid:11699058 |
| Nguyen TT et al. | Cholesterol-lowering effect of stanol ester in a US population of mildly hypercholesterolemic men and women: a randomized controlled trial. | 1999 | Mayo Clin. Proc. | pmid:10593347 |
| Hallikainen M et al. | Cholesterol metabolism and serum non-cholesterol sterols: summary of 13 plant stanol ester interventions. | 2014 | Lipids Health Dis | pmid:24766766 |
| Mo S et al. | Quantitative analysis of phytosterols in edible oils using APCI liquid chromatography-tandem mass spectrometry. | 2013 | Lipids | pmid:23884629 |
| Forchielli ML et al. | The spectrum of plant and animal sterols in different oil-derived intravenous emulsions. | 2010 | Lipids | pmid:20049583 |
| Lupattelli G et al. | Cholesterol metabolism differs after statin therapy according to the type of hyperlipemia. | 2012 | Life Sci. | pmid:22554491 |
| Barbosa SP et al. | Effects of ezetimibe on markers of synthesis and absorption of cholesterol in high-risk patients with elevated C-reactive protein. | 2013 | Life Sci. | pmid:23507424 |
| Tilvis RS et al. | Cholesterol and all-cause mortality in Honolulu. | 2001 | Lancet | pmid:11741659 |
| Nasu K et al. | Impact of cholesterol metabolism on coronary plaque vulnerability of target vessels: a combined analysis of virtual histology intravascular ultrasound and optical coherence tomography. | 2013 | JACC Cardiovasc Interv | pmid:23769651 |
| Sharma M et al. | Inhibition of sterol biosynthesis reduces tombusvirus replication in yeast and plants. | 2010 | J. Virol. | pmid:20015981 |
| Weingärtner O et al. | Plant sterol ester diet supplementation increases serum plant sterols and markers of cholesterol synthesis, but has no effect on total cholesterol levels. | 2017 | J. Steroid Biochem. Mol. Biol. | pmid:27473562 |
| Stellaard F et al. | The value of surrogate markers to monitor cholesterol absorption, synthesis and bioconversion to bile acids under lipid lowering therapies. | 2017 | J. Steroid Biochem. Mol. Biol. | pmid:27060336 |
| Clément C et al. | Influence of colour type and previous cultivation on secondary metabolites in hypocotyls and leaves of maca (Lepidium meyenii Walpers). | 2010 | J. Sci. Food Agric. | pmid:20355123 |
| Pakarinen MP et al. | Growth hormone selectively improves intestinal cholesterol absorption after jejunoileal autotransplantation in pigs. | 2004 | J. Pediatr. Surg. | pmid:15300531 |
| Kurvinen A et al. | Effects of long-term parenteral nutrition on serum lipids, plant sterols, cholesterol metabolism, and liver histology in pediatric intestinal failure. | 2011 | J. Pediatr. Gastroenterol. Nutr. | pmid:21543999 |
| Kurvinen A et al. | Parenteral plant sterols and intestinal failure-associated liver disease in neonates. | 2012 | J. Pediatr. Gastroenterol. Nutr. | pmid:22197940 |
| Hedman M et al. | Serum noncholesterol sterols in children with heterozygous familial hypercholesterolemia undergoing pravastatin therapy. | 2006 | J. Pediatr. | pmid:16492436 |
| Awad AB et al. | Effect of phytosterols on cholesterol metabolism and MAP kinase in MDA-MB-231 human breast cancer cells. | 2003 | J. Nutr. Biochem. | pmid:12667603 |
| Gorinstein S et al. | Comparison of the contents of the main biochemical compounds and the antioxidant activity of some Spanish olive oils as determined by four different radical scavenging tests. | 2003 | J. Nutr. Biochem. | pmid:12742543 |
| Sabeva NS et al. | Phytosterols differentially influence ABC transporter expression, cholesterol efflux and inflammatory cytokine secretion in macrophage foam cells. | 2011 | J. Nutr. Biochem. | pmid:21111593 |
| Delaney B et al. | Oral absorption of phytosterols and emulsified phytosterols by Sprague-Dawley rats. | 2004 | J. Nutr. Biochem. | pmid:15135153 |
| Awad AB et al. | beta-Sitosterol stimulates ceramide metabolism in differentiated Caco2 cells. | 2005 | J. Nutr. Biochem. | pmid:16098730 |
| Fransen HP et al. | Customary use of plant sterol and plant stanol enriched margarine is associated with changes in serum plant sterol and stanol concentrations in humans. | 2007 | J. Nutr. | pmid:17449596 |
| Tomoyori H et al. | Phytosterol oxidation products are absorbed in the intestinal lymphatics in rats but do not accelerate atherosclerosis in apolipoprotein E-deficient mice. | 2004 | J. Nutr. | pmid:15226455 |
| Tammi A et al. | Dietary plant sterols alter the serum plant sterol concentration but not the cholesterol precursor sterol concentrations in young children (the STRIP Study). Special Turku Coronary Risk Factor Intervention Project. | 2001 | J. Nutr. | pmid:11435511 |
| van der Made SM et al. | Consuming a buttermilk drink containing lutein-enriched egg yolk daily for 1 year increased plasma lutein but did not affect serum lipid or lipoprotein concentrations in adults with early signs of age-related macular degeneration. | 2014 | J. Nutr. | pmid:24991045 |
| Khabazian I et al. | Isolation of various forms of sterol beta-D-glucoside from the seed of Cycas circinalis: neurotoxicity and implications for ALS-parkinsonism dementia complex. | 2002 | J. Neurochem. | pmid:12153476 |
| Jakulj L et al. | Plasma plant sterols serve as poor markers of cholesterol absorption in man. | 2013 | J. Lipid Res. | pmid:23178226 |
| Nes WR et al. | Steric effects at C-20 and C-24 on the metabolism of sterols by Tetrahymena pyriformis. | 1981 | J. Lipid Res. | pmid:6793681 |
| Relas H et al. | Fate of intravenously administered squalene and plant sterols in human subjects. | 2001 | J. Lipid Res. | pmid:11369807 |
| Pinedo S et al. | Plasma levels of plant sterols and the risk of coronary artery disease: the prospective EPIC-Norfolk Population Study. | 2007 | J. Lipid Res. | pmid:17074925 |